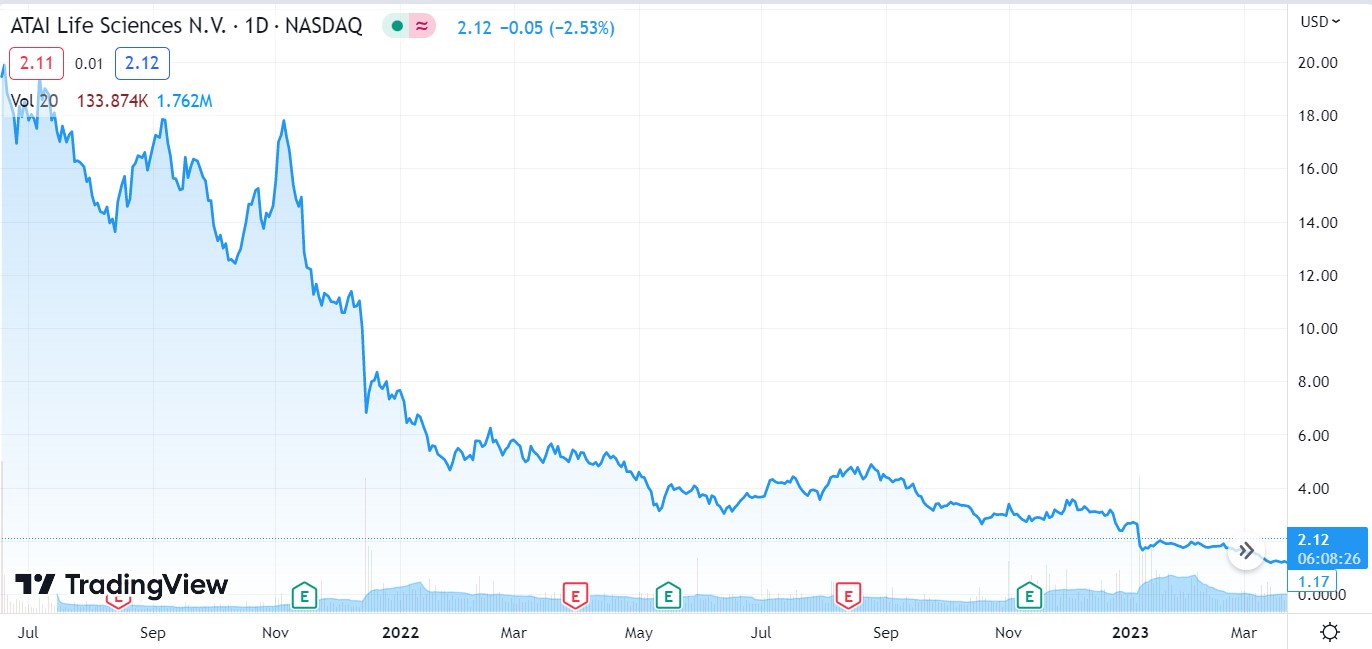
After dropping 94% from post IPO high from $19.45, a loss exceeding $3 billion and change, ATAI is in the early stages of recovery (in our opinion) gaining 94% since its low in January of 2023.
We were a little early, but picking bottoms is notoriously difficult. ATAI could gain 100% from its recent low of $1.14, before most investors even notice.
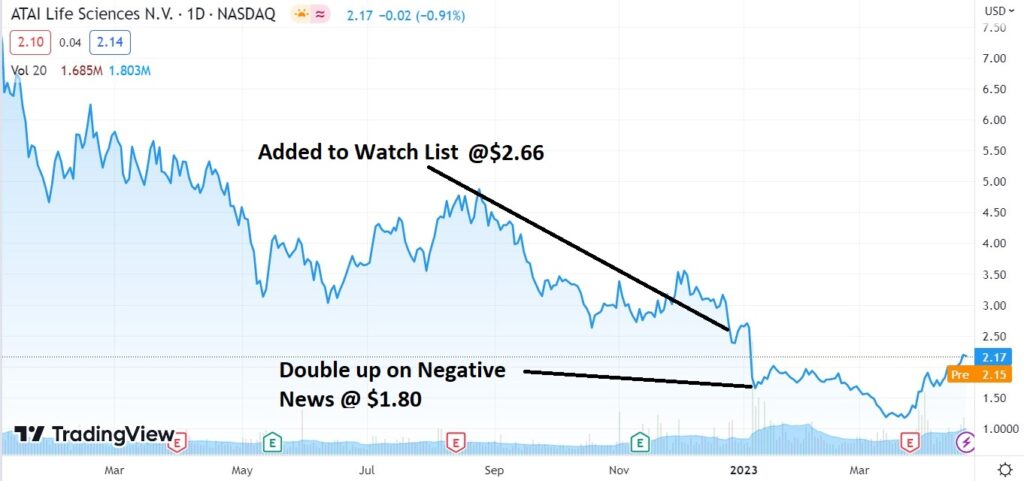
A revival in ATAI’s share price is of dramatic significance to the group as a whole. Which is more important to us than anything else.
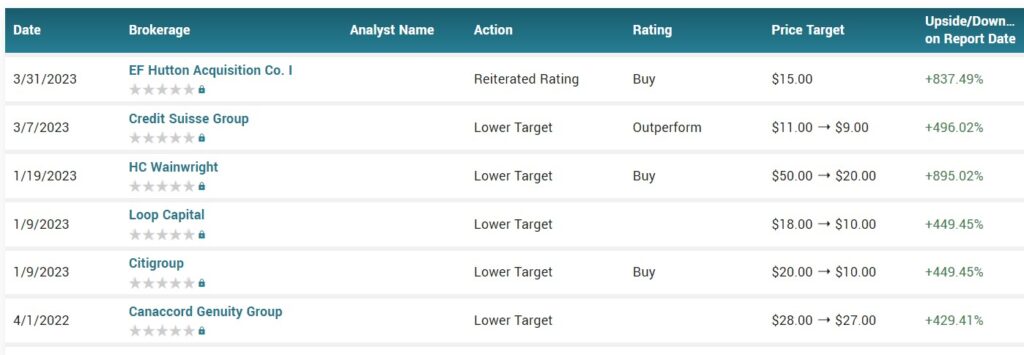
The most recent news of importance, after disastrous results from the FDA in early January, was that HC Wainwright’s Patrick Trucchio reduced his price target from $50 to $20. Can you believe it, a $20 price target?
We’d be thrown in jail if we even thought about making a projection like that – LOL. We’re regulated to say at most, “..we think it could do well from here!”
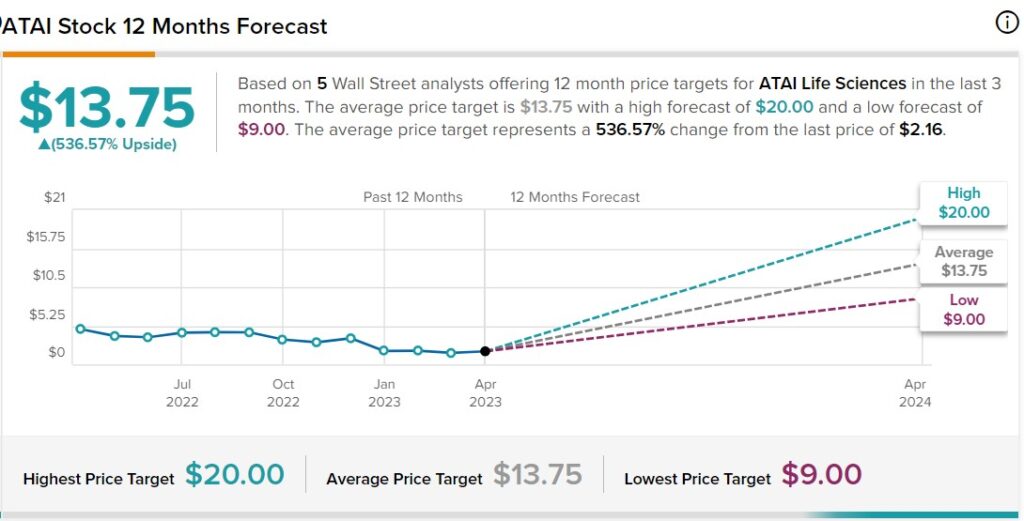
PRICE TARGETS
It would be pretty sad if these analysts are only ‘half’ right and investors miss a five-fold gain. It’s not like we didn’t tell you…
At this price level, ATAI should of interest to flippers, traders and long-term investors (us).
RECENT NEWS
Doubling Down After ATAI’s (ATAI) Anti Depression Treatment Bombs in Front of FDA.
HC Wainwright Analyst Still Has Confidence In Atai Life Sciences’ Pipeline Despite Recent Setbacks.
Even with a $20 price target, we caution investors to curb their enthusiasm (no margin, no home equity line of credit).
Yes the $360 million market cap is a long way from its former $3.3 billion – yet dangers remain. Best to play it by ear with no reason to take a double, so long as they continue to show progress with the FDA, while the share price continues to drift higher.
The company has many shots on goal (and $190 million in the bank), success with just one treatment could create dramatic results for the share price and for the sector — something which we are counting on.
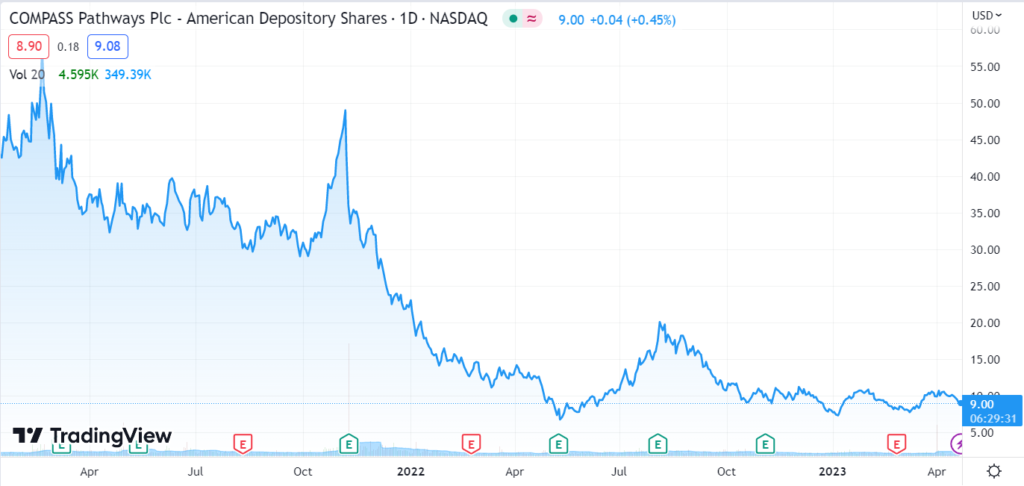
The other Company on our Watch List which can re-energize the entire sector is Compass Pathways (CMPS), which has gained 36% from its recent low.
Immunomedics (IMMU) $3.00 to $88, Up 29-fold, Our Second Best Idea Ever.
Atai Life Sciences N.V., through its subsidiary, ATAI Life Sciences AG, operates as a clinical-stage biopharmaceutical company.
It engages in developing various therapeutic candidates that focuses on various mental health disorders.
ATAI”s therapeutic candidates include PCN-101, a subcutaneous formulation of R-ketamine for the treatment of treatment-resistant depression (TRD); RL-007, a cholinergic, glutamatergic, and GABA-B receptor modulator to treat cognitive impairment associated with schizophrenia; DMX-1002, an oral formulation of ibogaine for treating opioid use disorder (OUD); GRX-917, an oral formulation of a deuterated version of etifoxine for the generalized anxiety disorder treatment; and NN-101, a novel intranasal formulation of N-acetylcysteine to treat mild traumatic brain injuries.
Its therapeutic candidates also comprise VLS-01, a formulation of N,N-dimethyltryptamine for treating TRD; EMP-01, an oral formulation of an 3,4-methyl enedioxy methamphetamine for the treatment of post-traumatic stress disorder; RLS-01, a formulation of Salvinorin A for TRD; KUR-101, an oral formulation of deuterated mitragynine to treat OUD; and DMX-1001, an oral formulation of noribogaine for the treatment of OUD.
The company was formerly known as Adripa Holding B.V. Atai Life Sciences N.V. was founded in 2018 and is headquartered in Berlin, Germany.
Disclaimer
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements contained in this press release other than statements of historical fact should be considered forward-looking statements, including without limitation statements regarding the success, cost, and timing of development of PCN-101 (R-ketamine) and related studies; our business strategy and plans, including potential partnerships and other strategic arrangements; and the plans and objectives of management for future operations and capital expenditures.
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are neither promises nor guarantees, and are subject to a number of important factors that could cause actual results to differ materially from any future results, performance or achievements expressed or implied by the forward-looking statements, including without limitation: we are a clinical-stage biopharmaceutical company and have incurred significant losses since our inception, and we expect to incur losses for the foreseeable future and may never be profitable; if we are unable to obtain funding when needed and on acceptable terms, we could be forced to delay, limit or discontinue our product development efforts; our limited operating history may make it difficult to evaluate the success of our business and to assess our future viability; we rely on third parties to assist in conducting our clinical trials and some aspects of our research and preclinical testing, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research, or testing; we currently rely on qualified therapists working at third-party clinical trial sites to administer certain of our product candidates in our clinical trials and we expect this to continue upon approval, if any, of our current or future product candidates, and if third-party sites fail to recruit and retain a sufficient number of therapists or effectively manage their therapists, our business, financial condition and results of operations would be materially harmed; our product candidates are in preclinical or clinical development, which is a lengthy and expensive process with uncertain outcomes, and we cannot give any assurance that any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized; research and development of drugs targeting the central nervous system, or CNS, is particularly difficult, and it can be difficult to predict and understand why a drug has a positive effect on some patients but not others; the production and sale of our product candidates may be considered illegal or may otherwise be restricted due to the use of controlled substances, which may also have consequences for the legality of investments from foreign jurisdictions; we face significant competition in an environment of rapid technological and scientific change, and there is a possibility that our competitors may achieve regulatory approval before we do or develop therapies that are safer, more advanced or more effective than ours, which may negatively impact our ability to successfully market or commercialize any product candidates we may develop and ultimately harm our financial condition; if we are unable to obtain and maintain sufficient intellectual property protection for our existing product candidates or any other product candidates that we may identify, or if the scope of the intellectual property protection we currently have or obtain in the future is not sufficiently broad, our competitors could develop and commercialize product candidates similar or identical to ours, and our ability to successfully commercialize our existing product candidates and any other product candidates that we may pursue may be impaired; third parties may claim that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and may prevent or delay our development and commercialization efforts; our future success depends on our ability to retain key employees, directors, consultants and advisors and to attract, retain and motivate qualified personnel; as a result of covenants to our loan agreement with Hercules Capital, Inc., our operating activities may be restricted and we may be required to repay the outstanding indebtedness in the event of a breach by us, or an event of default thereunder, which could have a materially adverse effect on our business; if we fail to maintain an effective system of disclosure controls and internal control over financial reporting our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired; our business is subject to economic, political, regulatory and other risks associated with international operations; a pandemic, epidemic, or outbreak of an infectious disease, such as the COVID-19 pandemic, may materially and adversely affect our business, including our preclinical studies, clinical trials, third parties on whom we rely, our supply chain, our ability to raise capital, our ability to conduct regular business and our financial results, and other risks, uncertainties, and assumptions described under “Risk Factors” in Item 1A of Part I, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of Part II and elsewhere in our Form 10-K for the year ended December 31, 2022, filed with the Securities and Exchange Commission.
Any forward-looking statements made herein speak only as of the date of this press release, and you should not rely on forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, performance, or achievements reflected in the forward-looking statements will be achieved or will occur. Except as required by applicable law, we undertake no obligation to update any of these forward-looking statements for any reason after the date of this press release or to conform these statements to actual results or revised expectations.
Contact Information
Investor Contact:
Stephen Bardin
Chief Financial Officer
[email protected]
Media Contact:
Allan Malievsky
Senior Director, External Affairs
[email protected]