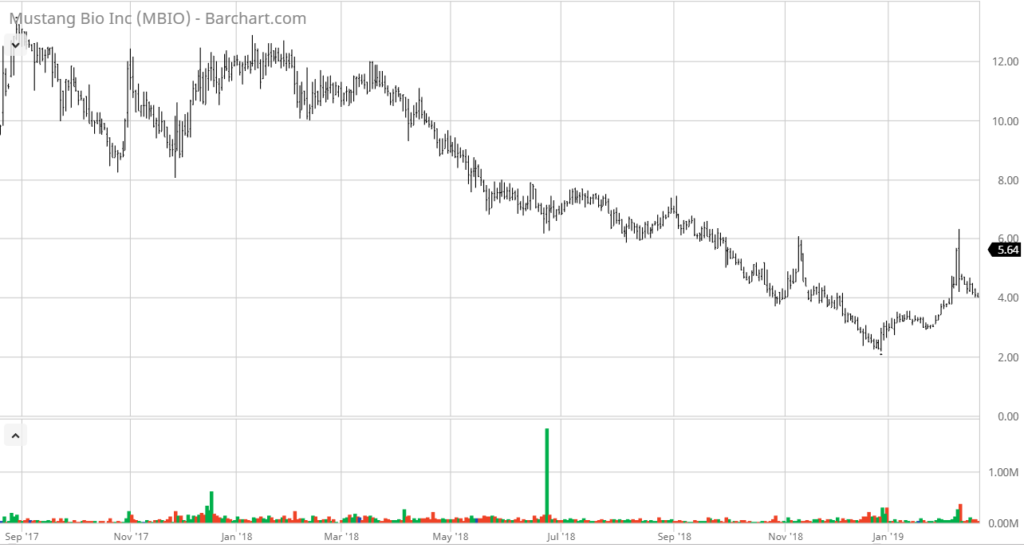
We met with management at Company-sponsored lunch at Ruth Chris in Boca Raton last week and we came away extremely enthusiastic about the Company’s prospects – and with the share price remaining near less than half 1/2 of the IPO price – the stars may be aligning here. We could be wrong, but it looks like there’s support at the $4.00 level.
The CEO Manny Litchman did an excellent job at explaining the technology in plain English at the investor meeting, to an audience who have large portfolios but do not have scientific backgrounds.
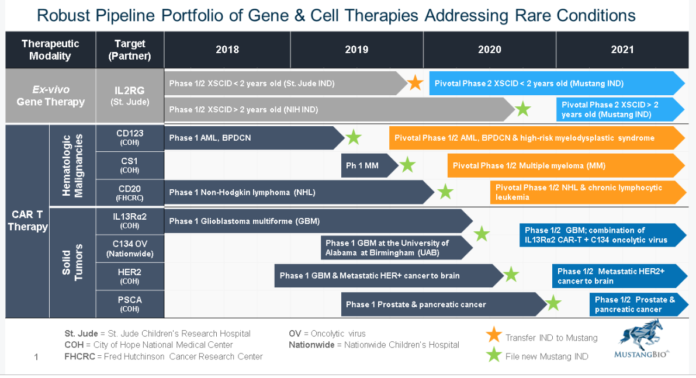
Yesterday Mustang announced it intends to combine the oncolytic virus (C134) with MB-101 (IL13Rα2-specific CAR) to potentially enhance efficacy in treating glioblastoma multiforme.
Announces an Exclusive Worldwide License Agreement for Oncolytic Virus (C134) to Treat Glioblastoma Multiforme with Nationwide Children’s Hospital.
Manuel Litchman, M.D., President and Chief Executive Officer of Mustang, said, “We are very pleased to partner with Nationwide Children’s Hospital to develop oncolytic virus C134. We also plan to evaluate oncolytic virus C134 in combination with MB-101 to explore the potential synergies of this novel combination to treat patients with glioblastoma. At Mustang, we are committed to evaluating our CAR T therapies alone and in combination regimens with the goal of advancing treatment paradigms for cancers without alternate therapies.”
In August of 2018, Mustang also announced that it entered into an exclusive worldwide license agreement with St. Jude Children’s Research Hospital for the development of a potentially first-in-class ex vivo lentiviral gene therapy for the treatment of XSCID, also known as bubble boy disease. The therapy is currently being evaluated in a Phase 1/2 multicenter trial in infants under the age of two. This study is the world’s first lentiviral gene therapy trial for infants with XSCID. The therapy is also being investigated in patients over the age of two in a second Phase 1/2 trial at the National Institutes of Health (“NIH”). The company believes these may be registration trials.
More details to come, please start your due diligence efforts here reading the recent offering prospectus.
Mustang BioMustang Bio, Inc. (“Mustang”), a Fortress Biotech (NASDAQ: FBIO) company, is a clinical‐stage biopharmaceutical company focused on the development and commercialization of a broad range of proprietary chimeric antigen receptor engineered T cell (CAR T) immunotherapies and gene therapies in areas of unmet need. Mustang aims to acquire rights to these technologies by licensing or otherwise acquiring an ownership interest, to fund research and development, and to outlicense or bring the technologies to market. Mustang has partnered with top medical institutions to advance the development of CAR T and CRISPR/Cas9-enhanced CAR T therapies across multiple cancers, as well as a lentiviral gene therapy for X-SCID. Mustang is registered under the Securities Exchange Act of 1934, as amended, and files periodic reports with the U.S. Securities and Exchange Commission. For more information, visit MustangBio.com.