Are we happy, you betcha!
We just got back from St Croix where the team from the Beverage Stock Review visited the Mutiny Island Vodka Distillery to see if it was true that the best tasting Vodka is made not from wheat, corn or potatoes, but from breadfruit. And its is true, it is simply the best. See crowdfunding site below.
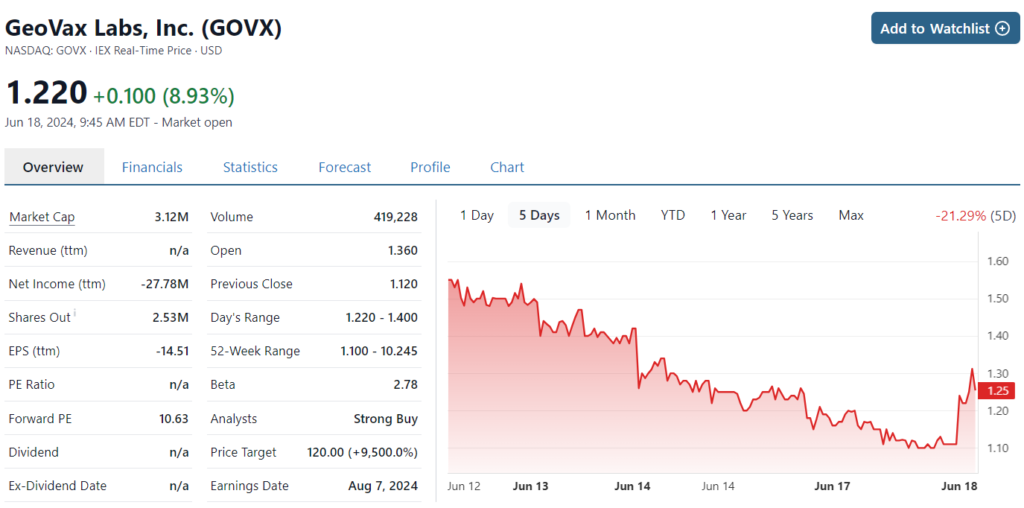
Then we come back to this news from BARDA. Are we happy? You betcha and Ya Mon. There is simply no telling where this could be a year from now. We feel rather certain though we’re looking at a 10-bagger, if they get FDA approval. Noble has a $6 price target followed by an $8 price target today from HC Wainwright.
Who needs Wall Street, when you have the US Government giving a non-dilutive GRANT, that provides up to $367 million in funding for the Phase 2b trial.
GeoVax Receives BARDA’S Project NextGen Award to Conduct Phase 2b Clinical Study Evaluating the Company’s Next-Generation COVID-19 Vaccine Candidate, GEO-CM04S1
What’s better than a recommendation from Goldman Sachs or JP Morgan? When the staff and team of experts at National Institute of Allergy and Infectious Diseases (NIAID) study GeoVax’s vaccine platform for months on end – and then give a recommendation to BARDA to fund it. It greatly increases the odds (though not guaranteed) that the vaccine does ultimately get approved by the FDA.
BARDA for your information has had 89 products approved by the FDA!
TEAM BARDA

Without a partner, GeoVax would have needed to dilute itself to the moon and back (which like with all biotech’s, we were okay with) to pay for a 10,000 patient clinical trial. No more. Now they have the US government as a partner. Wait, what, say that again?
The trial is designed as a Phase 2b double-blind study comparing GeoVax CM-04S1 “EmVeeAye” to an approved mRNA COVID-19 vaccine. Enrollment will have an estimated 10,000 healthy volunteers to determine efficacy, safety, and immunogenicity of the vaccines.
Previous trials with CM04S1 have shown both humoral and cellular immunity to multiple strains of the SARS-CoV-2 virus, with rapid and durable protection.
As a reminder, GeoVax is in three trials which are running concurrently. Two for the immunocompromised and one for the healthy.
So there is of course a chance for more happy unexpected news. Such as early (or expedited) approval, or a partnership with a public Pharma giant to fund the immunocompromised trials, because the vaccine is so badly needed by those patients. The mRNA’s have been said not to work very well with the immunocompromised.
And while most healthy people care not to take the vaccine these days, people with weakened immune systems DO NOT have that choice, they MUST be protected. Thus opening a huge lane and revenue generating opportunity for GeoVax. Huge.
Also as of this morning EVERY vaccine maker like Pfizer (PFE), Moderna (MRNA), Novavax (NVAX), and Sanofi (SNY) who have billions to invest, have now heard of GeoVax. We’re not going to say they’re running scared – but Pfizer is probably scared that Moderna is looking at GeoVax and Moderna is probably scared of Sanofi looking at GeoVax and so on…this is how it works in big Pharma land.
(PS We don’t see the point of continuing the healthy patient trial now that BARDA has arrived. We’ll do an interview with management and ask.)
Subscribe for our upcoming report which delves, not into the science – but into the math ($) of how much money GeoVax could potentially make if it does get approved by the FDA. And is then administered to some 23 million people in the US who are classified as immunocompromised (weakened immune systems), due to the drugs they are taking or post-transplant.
The following is an excerpt from the BARDA website:
Through Project NextGen, NIAID aims to accelerate the development of the next generation of COVID-19 vaccines that are ready for clinical evaluation.
NIAID plans to leverage existing infrastructure and network sites to implement a structured program evaluating up to 10 next generation COVID-19 vaccines in Phase 1 and Phase 2 clinical trials. The studies will be sponsored by NIAID using common protocols and centralized assays of multiple immune parameters including humoral, cellular, and mucosal responses. GeoVax is now one of them deemed worthy. What does that tell you?
These studies will help advance products as well as advance science in the field by evaluating how immunologic parameters correlate with protection from infection and disease.
Next generation vaccines include those with enhanced breadth of protection to variants, improved durability, and those with an enhanced ability to block infection/transmission including mucosal vaccines, relative to currently approved vaccines.
(Oh and yes Pfizer and Moderna and the other mRNA’s are well aware BARDA is trying to end-run them with this well funded project.)
NIAID is interested in working with vaccine manufacturers with next generation novel vaccine candidates currently at a development stage ready to enter clinical trials or capable of entering clinical trials by mid-2024.
So think about it. There are hundreds of vaccine candidates out there, and they picked GeoVax as one of the top 10. Kudos to management.
David Dodd CEO, Post on Linkedin.
Killer, 44-Page Report on GeoVax (GOVX).
Wall Street GeoVax (GOVX) Research Reports Archive
Noble Securities Update ($6.00 price target.)
HC Wainwright Reiterates ($8 price target)
Full Press Release:
Mutiny Island Vodka on Start Engine

Forward-Looking Statements
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.
Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law. Progress reporting and news coverage client, see report for full disclosure and disclaimer details.














