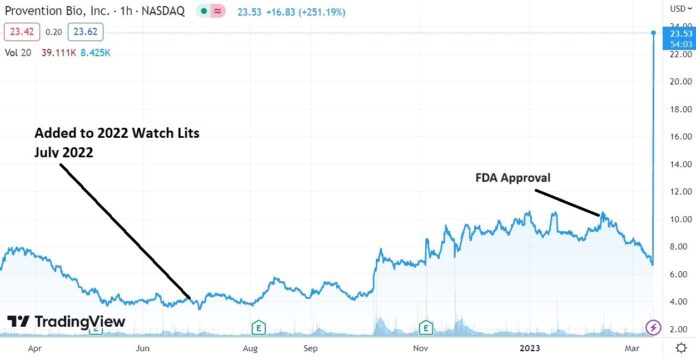
Up 416%, from Adding it to 2022 Watch List!
Great way to wake up after a night of hard partying at the C-3.
How pray tell, how did we make more than 400% in less than a year? A single one-day, $1.6 billion gain and payday for shareholders.
Paris and Red Bank, N.J. March 13, 2023 Sanofi and Provention Bio, Inc., a U.S.-based, publicly traded biopharmaceutical company focused on intercepting and preventing immune-mediated diseases including type 1 diabetes (T1D), have entered into an agreement under which Sanofi has agreed to acquire Provention Bio, Inc., for $25.00 per share in cash, representing an equity value of approximately $2.9 billion.
Sanofi to acquire Provention Bio for $2.9 billion.
Let’s thank Robert DePhillipis – our resident Biotech guru. He also gave us the Immunomedics (IMMU) idea which went from $2.50 to $87 up a whopping 2,385%.
Another round of drinks for Bobby!
And don’t say we didn’t tell ya.
Immunomedics (IMMU) $3.00 to $88, Up 29-fold, Our Second Best Idea Ever.
Sanofi to acquire Provention Bio, adding to portfolio TZIELD, the first disease-modifying treatment for the delay of Stage 3 type 1 diabetes (T1D)
Paris and Red Bank, N.J. March 13, 2023 Sanofi and Provention Bio, Inc., a U.S.-based, publicly traded biopharmaceutical company focused on intercepting and preventing immune-mediated diseases including type 1 diabetes (T1D), have entered into an agreement under which Sanofi has agreed to acquire Provention Bio, Inc., for $25.00 per share in cash, representing an equity value of approximately $2.9 billion.
The transaction adds an innovative, fully owned, first-in-class therapy in type 1 diabetes to Sanofi’s core asset portfolio in General Medicines and further drives its strategic shift toward products with a differentiated profile. TZIELD (teplizumab-mzwv) was approved in the U.S. last year as the first and only therapy to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
The acquisition is a strategic fit for Sanofi at the intersection of the company’s growth in immune-mediated diseases and disease-modifying therapies in areas of high unmet need, and its expertise in diabetes. Sanofi will continue to utilize its capabilities in diabetes to maximize TZIELD’s potential as a transformative therapy globally and in the U.S., aiming to delay the onset of Stage 3 type 1 diabetes for some of the approximately 65,000 people diagnosed every year1. The purchase builds on an existing co-promotion agreement with Provention Bio that is already delivering TZIELD to patients in need of this immune-mediated therapy.
Olivier Charmeil
Executive Vice President, General Medicines, Sanofi
“The acquisition of Provention Bio builds on Sanofi’s mission to deliver best- and first-in-class medicines and resonates with our purpose of chasing the miracles of science for the benefit of people. By coupling Provention Bio’s transformative innovation with Sanofi’s expertise, we aim to bring life-changing benefits to people at risk of developing Stage 3 type 1 diabetes. Any additional indications, approvals and pipeline assets only serve to further our excitement. Given our existing partnership and complementary work in the diabetes and immunology spaces, we foresee a seamless integration and execution.”
TZIELD: First and only treatment indicated to delay onset of Stage 3 T1D
TZIELD is a CD3-directed antibody indicated to delay the onset of Stage 3 T1D in adults and pediatric patients aged 8 years and older with Stage 2 T1D. Stage 3 T1D is associated with significant health risks, including diabetic ketoacidosis, which can be life threatening, and patients who progress to Stage 3 T1D eventually require insulin injections for life.
TZIELD is also in late-stage clinical development for the treatment of pediatric and adolescent patients that are newly diagnosed with clinical T1D (Stage 3). A Phase 3 trial, PROTECT, is currently underway and top line results are expected in the second half of 2023. Additional opportunities for TZIELD include re-dosing and formulations as well as new therapeutic indications.
Ashleigh Palmer
Chief Executive Officer and Co-Founder, Provention Bio, Inc.
“Sanofi and Provention Bio share a common vision of bringing new therapies to patients with autoimmune diseases. Under our co-promotion agreement, our companies have made significant progress educating healthcare providers and increasing patient access during the initial U.S. commercial launch of TZIELD. Sanofi’s global expertise and commitment to immunology makes them an ideal acquiror and positions our innovative therapy to reach more patients as quickly as possible.”
Provention Bio also brings certain pipeline assets in early development in immune-mediated diseases.
Transaction Terms
Under the terms of the merger agreement, Sanofi will commence a cash tender offer to acquire all outstanding shares of Provention Bio, Inc. for $25.00 per share in cash, reflecting a total equity value of approximately $2.9 billion.
The consummation of the tender offer is subject to customary closing conditions, including the tender of a number of shares of Provention Bio, Inc. common stock, that together with shares already owned by Sanofi or its affiliates, represents at least a majority of the outstanding shares of Provention Bio, Inc. common stock, the expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, and other customary conditions.
If the tender offer is successfully completed, then following the successful completion of the tender offer, a wholly owned subsidiary of Sanofi will merge with and into Provention Bio, Inc., and all of the outstanding Provention Bio, Inc. shares that are not tendered in the tender offer will be converted into the right to receive the same $25.00 per share in cash offered to Provention Bio, Inc. shareholders in the tender offer. Sanofi plans to fund the transaction with available cash resources. Subject to the satisfaction or waiver of customary closing conditions, Sanofi currently expects to complete the acquisition in the second quarter of 2023.
PJT Partners is acting as exclusive financial advisor to Sanofi and Weil, Gotshal & Manges LLP is acting as its legal counsel. BofA Securities, Inc. and Centerview Partners LLC are acting as financial advisors to Provention Bio, Inc. and Ropes & Gray LLP is acting as its legal counsel.
About Sanofi
We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives. Our team, across some 100 countries, is dedicated to transforming the practice of medicine by working to turn the impossible into the possible. We provide potentially life-changing treatment options and life-saving vaccine protection to millions of people globally, while putting sustainability and social responsibility at the center of our ambitions.
Sanofi is listed on EURONEXT: SAN and NASDAQ: SNY
About Provention Bio, Inc.
Provention Bio, Inc. is a commercial-stage biopharmaceutical company focused on advancing the development and commercialization of investigational therapies that may intercept and prevent debilitating and life-threatening immune-mediated diseases. The Company’s pipeline includes clinical-stage product candidates that have demonstrated in pre-clinical or clinical studies proof-of-mechanism and/or proof-of-concept in autoimmune diseases, including T1D, celiac disease and lupus. Visit www.proventionbio.com for more information and follow on Twitter: @ProventionBio.
Provention Bio, Inc. is listed on Nasdaq: PRVB
About T1D
Type 1 diabetes is a condition caused by autoimmune damage of the insulin-producing beta-cells of the pancreas. As a result of this autoimmune attack, the body produces very little or no insulin which can lead to death if the insulin is not replaced.
Living with T1D is complex. In addition to daily insulin injections or infusion via an insulin pump, people living with T1D also need to adopt a strict management plan which includes regular blood sugar monitoring, healthy diet and physical activity.
Media Relations
Sandrine Guendoul | + 33 6 25 09 14 25 | sandrine.guendoul@sanofi.com
Evan Berland | + 1 215 432 0234 | evan.berland@sanofi.com
Nicolas Obrist | + 33 6 77 21 27 55 | nicolas.obrist@sanofi.com
Sally Bain | + 1 617 834 6026 | sally.bain@sanofi.com
Investor Relations
Eva Schaefer-Jansen | + 33 7 86 80 56 39 | eva.schaefer-jansen@sanofi.com
Arnaud Delépine | + 33 6 73 69 36 93 | arnaud.delepine@sanofi.com
Corentine Driancourt | + 33 6 40 56 92 21 | corentine.driancourt@sanofi.com
Felix Lauscher | + 1 908 612 7239 | felix.lauscher@sanofi.com
Tarik Elgoutni | + 1 617 710 3587 | tarik.elgoutni@sanofi.com
Nathalie Pham | + 33 7 85 93 30 17 | nathalie.pham@sanofi.com
Provention Bio, Inc. Investor Relations
Kristen Keller | investorrelations@proventionbio.com
Sanofi Forward-Looking Statements2
This press release contains forward-looking statements. Forward-looking statements are statements that are not historical facts. These statements may include projections and estimates regarding the marketing and other potential of the product, or regarding potential future revenues from the product, and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “will be”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, unexpected regulatory actions or delays, or government regulation generally, that could affect the availability or commercial potential of the product, the fact that product may not be commercially successful and risks related to Sanofi’s ability to complete the acquisition on the proposed terms or on the proposed timeline, including the receipt of required regulatory approvals, the possibility that competing offers will be made, other risks associated with executing business combination transactions, such as the risk that the businesses will not be integrated successfully, that such integration may be more difficult, time-consuming or costly than expected or that the expected benefits of the acquisition will not be realized, as well as other risks related Sanofi’s business, including the uncertainties inherent in research and development, including future clinical data and analysis of existing clinical data relating to the product, including post marketing, unexpected safety, quality or manufacturing issues, competition in general, risks associated with intellectual property and any related future litigation and the ultimate outcome of such litigation, and volatile economic and market conditions, and the impact that COVID-19 will have on us, our customers, suppliers, vendors, and other business partners, and the financial condition of any one of them, as well as on our employees and on the global economy as a whole. The risks and uncertainties also include the uncertainties discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2022. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Provention Bio, Inc. Forward-Looking Statements
This press release includes forward-looking statements that are subject to risks, uncertainties and other factors that could cause actual results to differ materially from those implied by the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including all statements regarding the intent, belief or current expectation of Provention Bio, Inc. (“Provention Bio, Inc.”) and members of its senior management team and can typically be identified by words such as “believe,” “expect,” “estimate,” “predict,” “target,” “potential,” “likely,” “continue,” “ongoing,” “could,” “should,” “intend,” “may,” “might,” “plan,” “seek,” “anticipate,” “project” and similar expressions, as well as variations or negatives of these words. Forward-looking statements include, without limitation, statements regarding the proposed transaction, prospective performance, future plans, events, expectations, performance, objectives and opportunities and the outlook for Provention Bio, Inc.’s business; the commercial success of Provention Bio, Inc.’s products and pipeline; the expected timing of the completion of the transaction; the ability to complete the transaction considering the various closing conditions; and the accuracy of any assumptions underlying any of the foregoing. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and are cautioned not to place undue reliance on these forward-looking statements. Actual results may differ materially from those currently anticipated due to a number of risks and uncertainties. Risks and uncertainties that could cause the actual results to differ from expectations contemplated by forward-looking statements include: uncertainties as to the timing of the tender offer and merger; uncertainties as to how many of Provention Bio, Inc.’s stockholders will tender their stock in the offer; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the transaction; the occurrence of any event, change or other circumstance that could give rise to the termination of the merger agreement; the effects of the transaction (or the announcement thereof) on relationships with associates, customers, manufacturers, suppliers, other business partners or governmental entities or patient groups; transaction costs; the risk that the merger will divert management’s attention from Provention Bio, Inc.’s ongoing business operations; changes in Provention Bio, Inc.’s businesses during the period between now and the closing; risks associated with litigation; failure to maintain FDA approval for TZIELD; uncertainties that the planned commercial launch in the U.S. for TZIELD is successful in part or at all for various reasons including the actual market size and drug supply needed may not be consistent with Provention Bio, Inc.’s expectations and its executed commercial readiness plans; uncertainties as to the degree to which TZIELD is accepted by patients and prescribed by physicians; uncertainties as to the efficiency of Provention Bio, Inc.’s manufacturing, sales, distribution and specialty pharmacy network in getting TZIELD to the market and future economic, competitive, reimbursement and regulatory conditions that could negatively impact the commercial launch of TZIELD; risks that the post-marketing commitment studies for TZIELD may not yield data consistent with prior results; the risk that TZIELD may cause undesirable side effects that could limit its commercial potential; the possibility that Provention Bio, Inc. is not able to execute on its business plans including meeting its expected or planned regulatory milestones and timelines, clinical development plans and successfully bringing its product candidates to market, for various reasons, including factors outside of Provention Bio, Inc.’s control, such as possible limitations of Provention Bio, Inc.’s financial and other resources, competition, manufacturing limitations that may not be anticipated or resolved for in a timely manner or at all, and regulatory, court or agency decisions, such as decisions by the United States Patent and Trademark Office with respect to patents that cover its product candidates, the potential for noncompliance with FDA regulations; the potential impacts of COVID-19 on Provention Bio, Inc.’s business and financial results; changes in law, regulations, or interpretations and enforcement of regulatory guidance; uncertainties of patent protection and litigation; competition, other risks and uncertainties detailed from time to time in documents filed with the SEC by Provention Bio, Inc., including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K, as well as the Schedule 14D-9 to be filed by Provention Bio, Inc.. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval, and commercialization of new products. All forward-looking statements are based on information currently available to Provention Bio, Inc., and Provention Bio, Inc. assumes no obligation to update any forward-looking statements, whether as a result of new information, future developments or otherwise, except as may be required by applicable law. The information set forth herein speaks only as of the date hereof.
Additional Information for US Shareholders and Where to Find It
The tender offer for the outstanding shares of common stock of Provention Bio, Inc. referenced in this communication has not yet commenced. This communication is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Provention Bio, Inc., nor is it a substitute for the tender offer materials that Sanofi and its acquisition subsidiary will file with the U.S. Securities and Exchange Commission (the “SEC”) upon commencement of the tender offer. At the time the tender offer is commenced, Sanofi and its acquisition subsidiary will file a tender offer statement on Schedule TO, and Provention Bio, Inc. will file a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer. The tender offer statement on Schedule TO (including an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents) and the Solicitation/Recommendation Statement will contain important information. HOLDERS OF SHARES OF PROVENTION BIO, INC. ARE URGED TO READ THESE DOCUMENTS WHEN THEY BECOME AVAILABLE (AS EACH MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME) BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION THAT PROVENTION BIO, INC. STOCKHOLDERS SHOULD CONSIDER BEFORE MAKING ANY DECISION REGARDING TENDERING THEIR SHARES. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, will be made available to all holders of shares of Provention Bio., Inc. at no expense to them. The tender offer materials and the Solicitation/Recommendation Statement will be made available for free at the SEC’s web site at www.sec.gov. Additional copies may be obtained for free by contacting Sanofi’s Investor Relations Department at ir@sanofi.com or on Sanofi’s website at https://en.sanofi.com/investors or by contacting Kristen Kelleher, Investor Relations, at investorrelations@proventionbio.com, or on Proventon Bio, Inc.’s website, www.proventionbio.com.
In addition to the Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, Sanofi files annual and special reports and other information with the SEC and Provention Bio., Inc. files annual, quarterly and special reports and other information with the SEC. You may read and copy any reports or other information filed by Sanofi and Provention Bio., Inc. at the SEC public reference room at 100 F. Street, N.E., Washington D.C. 20549. Please call the Commission at 1-800-SEC-0330 for further information on the public reference room. Sanofi’s and Provention Bio., Inc.’s filings with the SEC are also available to the public from commercial document-retrieval services and at the website maintained by the SEC at www.sec.gov
1 Based on Sanofi analysis derived from the 2020 CDC National Diabetes Statistics Report https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf