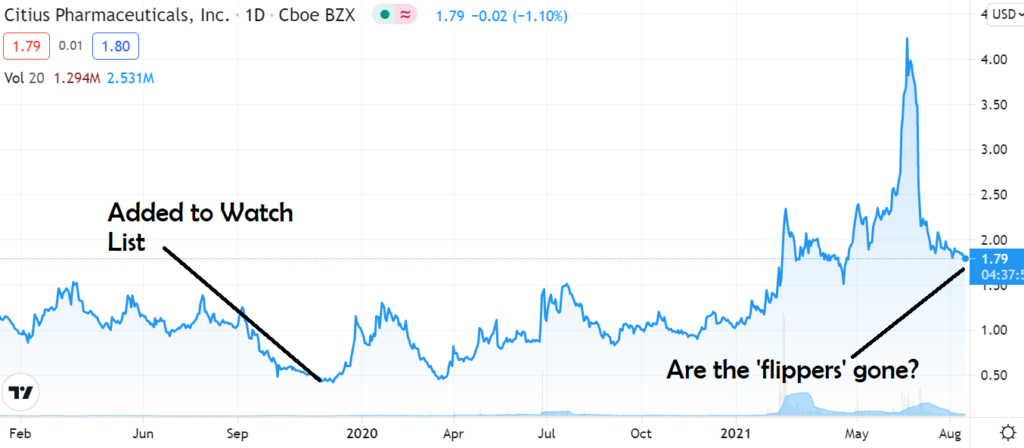
- Added to Watch List November 2019 @$0.55.
- Named Our Top Idea in 2020 @$1.02.
- Remains Our Top Idea in 2021 @$1.80
- Cash has Risen From $3.3 Million in 2019 to $115 Million on 2021.
Despite gaining 245% from where we initiated coverage in late 2019, Citius, in our opinion, on this day and at this price – has never looked better for long term investors. The flippers are gone. It’s game time folks.
This has never been a complex story with only two things of primary importance in our eyes. The first is, are they advancing their science of Mino-Lok® in the eyes of the FDA? The second is do they have the financial strength to continue to advance the science? Trials proving it’s safe and works takes money, and plenty of it.
ADVANCING THE SCIENCE
With regards to advancing the science, and while there is guarantee they’ll get Mino-Lok® approved, they are far into the approval process. Shareholders are not facing a ‘watch the paint dry,’ yet at the same time ‘nerve-wracking’ 5-10 year journey ahead.
Citius is in phase III with the latest ‘go-ahead’ recommendation coming July 1st, 2021.
In the words of the Company, “This recommendation affirms that there is an important efficacy signal that merits moving forward with the trial, there are no safety concerns to warrant halting the trial, and that the full data set upon trial completion may support statistically significant superiority.”
That was the third review recommendation they’ve received and the Company expects to complete the Mino-Lok® trial by the end of 2021 or early 2022, subject to continued easing of COVID-19 restrictions in the U.S..
Keep in mind, the successful Phase 2b was completed six years ago in December of 2014. Many things could have gone wrong in the past six years, yet nothing went wrong. The point we’re trying to make, is that investors will not have to wait another six years.
Here is an important study released back in 2016 for information junkies:
Successful Salvage of Central Venous Catheters.
FUNDING THE SCIENCE
This is simple. They have $115 million. When we initiated coverage they had less than $5 million. Total, nerve wracking. It was too risky for most investors (which is fine), but speculators who took the risk are now up 245%.
The mountain of cash means not only will they be able to fund the final trials of Mino-Lok® but they’ll also be able to fund other science in their pipeline. Citius is at the sweetest of its sweet-spots since we’ve been following it.
In an upcoming report we’ll delve into the other trials the funding will help advance including Mino-Wrap, Hal-Lido and Novecite’s proposed novel Stem Cell therapy for ARDS (yup Corona related).
On top of all that, while not identified as one Citius, is in fact a technology incubator. They are on a constant hunt to identify later-stage drug candidates that can be developed within a 3-4 year time horizon, using a 505(b)(2) pathway. And having $100 million in the bank, has many of the world’s top scientist’s knocking on their door.
Citius Pharmaceuticals, Inc. Reports Third Fiscal Quarter 2021 Financial Results and Provides General Business Update.
$115.7 million in cash and cash equivalents as of June 30, 2021 to develop pipeline and invest in long-term growth
Mino-Lok® Phase 3 superiority trial advancing following independent Data Monitoring Committee recommendation to proceed without any modifications
CRANFORD, N.J., Aug. 12, 2021 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) (Nasdaq: CTXR), a biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products with a focus on anti-infective products in adjunct cancer care, unique prescription products and stem cell therapy, today reported financial results for the third fiscal quarter of 2021 ended June 30, 2021, and provided a general business update.
Third Fiscal Quarter 2021 and Recent Business Highlights
Received third positive recommendation from independent Data Monitoring Committee to continue the Mino-Lok® Phase 3 clinical superiority trial as planned without modifications; no safety concerns identified;
Characterization and expansion of NoveCite’s i-MSC accession cell bank (ACB) underway at Waisman Biomanufacturing at the University of Wisconsin-Madison to create a cGMP master cell bank (MCB);
Received FDA guidance on proprietary Halo-Lido patient-reported outcome (PRO) tool; preparing to submit an investigational new drug (IND) application during the fourth quarter of 2021;
Advanced Mino-Wrap chemistry, manufacturing and controls (CMC) development;
Issued 11.2 million shares of Citius common stock upon the exercise of warrants during the quarter for aggregate proceeds of $16.9 million, and a total of $127.6 million in financing activities during the first half of 2021;
Announced that on June 21, 2021 stockholders approved an increase in authorized shares from 210,000,000 to 410,000,000 and an increase in authorized common shares from 200,000,000 to 400,000,000; and,
Reported $115.7 million in cash and cash equivalents as of June 30, 2021.
“During the quarter, we made progress with all of our programs despite the ongoing challenges of conducting preclinical work and clinical trials during the extended COVID-19 pandemic. We remain encouraged by the positive recommendation of the independent DMC to continue the Mino-Lok® Phase 3 pivotal superiority trial as planned and are fully committed to submitting an NDA for the treatment of infected catheters, a potentially life-threatening condition for the nearly 500,000 patients with catheter-related bloodstream infections in the U.S. each year. Given rising COVID-19 infection rates across the U.S., hospitals are, understandably, prioritizing COVID-19 patients and studies. Consequently, we expect recruitment for our Mino-Lok® trial will be slower in the near term than we had originally planned. To address this, we are exploring multiple paths to support our patient recruitment and randomization efforts and are confident that we have the resources in place to complete the trial in a timely manner. We look forward to updating you on these efforts in due course,” stated Myron Holubiak, President and Chief Executive Officer of Citius Pharmaceuticals.
“The persistence of COVID-19 is a reminder that treatment of acute respiratory distress syndrome (ARDS) will continue to be an important need worldwide. Accordingly, we remain focused on further developing our engineered stem cell program, which holds the potential to offer a novel and scalable therapy for all causes of ARDS. We have initiated a pilot study in mice and are completing our proof-of-concept sheep study, for which we expect to have topline results by the end of this quarter. Additionally, our IND submission for Halo-Lido is on track for later this year and in vitro CMC work for Mino-Wrap is underway. With a solid balance sheet to support our activities, we are now better positioned than ever before to execute our strategy and deliver value to patients and shareholders,” added Mr. Holubiak.
Third Fiscal Quarter 2021 Financial Results:
Liquidity
As of June 30, 2021, the Company had $115.7 million in cash and cash equivalents. During the three months ended June 30, 2021, the Company issued 11.2 million shares of Citius common stock upon the exercise of warrants for aggregate proceeds of approximately $16.9 million. During the nine months ended June 30, 2021, the Company received $31.1 million in proceeds from the exercise of common stock warrants.
In January 2021, the Company closed a private placement for common stock and warrants totaling gross proceeds of approximately $20 million. In February 2021, the Company closed a registered direct offering of its common stock and warrants for gross proceeds of approximately $76.5 million.
On June 21, 2021, stockholders approved an amendment to the Company’s Articles of Incorporation to increase the authorized number of shares from 210,000,000 to 410,000,000 and the authorized number of common shares from 200,000,000 to 400,000,000. As of June 30, 2021, the Company had 145,979,429 common shares issued and outstanding.
During the first half of 2021, the Company raised a total of $127.6 million through financing activities. We estimate that we will have sufficient funds for our operations through March 2023.
Research and Development (R&D) Expenses
R&D expenses were $2.2 million and $9.9 million for the three and nine months ended June 30, 2021, respectively, compared to $2.6 million and $7.3 million for the comparable periods in 2020. The decrease in research and development expenses for the quarter ended June 30, 2021, compared to the prior-year quarter reflects decreases in R&D expenses for our Mino-Lok® and Halo-Lido product candidates, offset by an increase in R&D expenses for Mino-Wrap and our proposed novel stem cell therapy for acute respiratory distress syndrome (ARDS).
The increase in research and development expenses for the nine months ended June 30, 2021 compared to the prior year period is primarily due to a $5.5 million increase in R&D expenses related to our proposed novel stem cell therapy for ARDS, offset by decreases in R&D expenses for our other pipeline products, including a decrease in manufacturing research and development costs for registration batches of Mino-Lok® and a decrease in R&D expenses associated with manufacturing development and our patient reported outcome tool for Halo-Lido.
We expect that research and development expenses will increase in fiscal 2021 as we continue to focus on our Phase 3 trial for Mino-Lok®, progress the Halo-Lido product candidate, and continue our research and development efforts related to ARDS and Mino-Wrap.
General and Administrative (G&A) Expenses
G&A expenses were $3.4 million and $7.4 million for the three and nine months ended June 30, 2021, respectively, compared to $1.9 million and $5.7 million for the comparable periods in 2020. The primary reason for the increase is incremental costs associated with investor relations and legal services, as well as additional compensation costs for new employees and performance bonuses. General and administrative expenses consist primarily of compensation costs, professional fees related to our capital raising activities, corporate development services, and investor relations.
Stock-based Compensation Expense
For the three months ended June 30, 2021, stock-based compensation expense was $0.4 million as compared to $0.2 million for the three months ended June 30, 2020. For the nine months ended June 30, 2021, stock-based compensation expense was $1.0 million as compared to $0.6 million for the nine months ended June 30, 2020. The increase reflects expenses related to new grants made by Citius and the recently adopted NoveCite stock option plan.
Net loss
Net loss was $5.8 million and $18.1 million for the three and nine months ended June 30, respectively, compared to a net loss of $4.7 million and $13.4 million for the comparable periods in 2020. The increase in net loss is primarily due to the increase in general and administrative expenses and an increase in our research and development activities related to ARDS.
About Citius Pharmaceuticals, Inc.
Citius is a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products, with a focus on anti-infectives in adjunct cancer care, unique prescription products, and stem cell therapy. The Company’s lead product candidate, Mino-Lok®, an antibiotic lock solution for the treatment of patients with catheter-related bloodstream infections (CRBSIs), is currently enrolling patients in a Phase 3 pivotal superiority trial. Mino-Lok® was granted Fast Track designation by the U.S. Food and Drug Administration (FDA). Through its subsidiary, NoveCite, Inc., Citius is developing a novel proprietary mesenchymal stem cell treatment derived from induced pluripotent stem cells (iPSCs) for acute respiratory conditions, with a near-term focus on acute respiratory distress syndrome (ARDS) associated with COVID-19. For more information, please visit www.citiuspharma.com.
Safe Harbor
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “plan,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: our ability to successfully undertake and complete clinical trials and the results from those trials for our product candidates; risks relating to the results of research and development activities; uncertainties relating to preclinical and clinical testing; the early stage of products under development; our need for substantial additional funds; the estimated markets for our product candidates and the acceptance thereof by any market; the ability of our product candidates to impact the quality of life of our target patient populations; market and other conditions; risks related to our growth strategy; patent and intellectual property matters; our ability to attract, integrate, and retain key personnel; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; our dependence on third-party suppliers; our ability to procure cGMP commercial-scale supply; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further impacted by Covid-19. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”) filings which are available on the SEC’s website at www.sec.gov, including in our Annual Report on Form 10-K for the year ended September 30, 2020, filed with the SEC on December 16, 2020 and updated by our subsequent filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law. Client see report for full disclosure and disclaimer details.
Investor Relations for Citius Pharmaceuticals:
Ilanit Allen
Vice President, Corporate Communications and Investor Relations
T: 908-967-6677 x113
E: [email protected]
$CTXR, #CTXR












