THOUSAND OAKS, Ca.–(BUSINESS WIRE)–Dyve Biosciences, Inc. today announced that it has received clearance from the U.S. Food and Drug Administration to proceed with a Phase 2 trial of DYV-700, the company’s topically administered therapeutic being developed to reduce the pain and duration of an acute gout flare.
Dyve intends to initiate the Phase 2 trial, the TARGETS Study (NCT04130204), in the first quarter of 2020. TARGETS is a double-blind, placebo-controlled, randomized, clinical trial to evaluate the efficacy of DYV-700 applied topically to patients with an acute gout flare. TARGETS is designed to enroll 300 subjects across 20 trial centers in the U.S. The primary objectives are to evaluate DYV-700 efficacy, defined as a reduction in pain intensity and a reduction in the duration of the gout attack. The clinical study protocol was developed in partnership with the Dyve Scientific Advisory Board, members of which are listed on the company’s website. The company plans to provide additional study details following its initiation.

Ryan Beal, M.D., chief executive officer at Dyve, said: “DYV-700 is the first therapeutic derived from our proprietary skin penetration platform technology to reach Phase 2 and represents a significant milestone for Dyve. More importantly, it represents potential hope for millions of gout patients who suffer from the excruciating pain of an acute gout flare. There has been very little innovation in acute gout and current treatment options take days to work and have an unfavorable adverse event profile. Our pilot study showed DYV-700 has the potential to introduce a paradigm shift in the treatment of acute gout.”
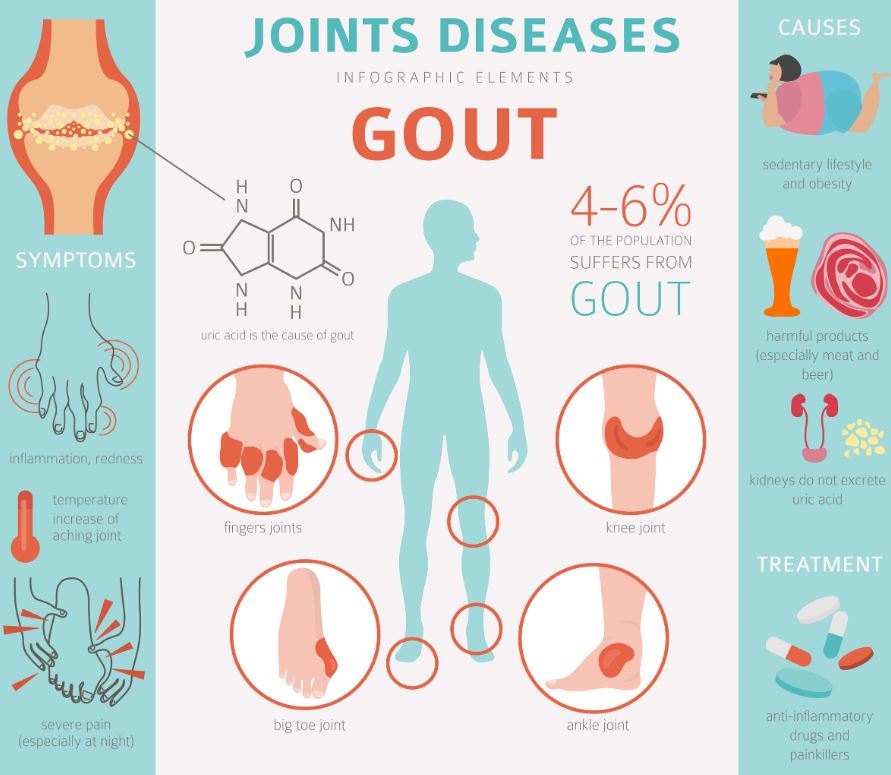
In the U.S., gout affects over 10 million people with more than 40% of patients having two or more attacks per year. The number of gout patients is growing due to poor chronic gout management and the condition’s links to hypertension, renal failure, diabetes, and obesity. In addition, the standard of care for an acute gout flare has been the same for nearly 40 years, works slowly, and is associated with GI distress in ~23% of patients.
RELATED: If Gout is a Disease of the Rich, Why Do I Have It?
Dyve’s technology allows an intuitive topical application to rapidly deliver DYV-700 through the skin and quickly dissolve pain-inducing monosodium urate crystals in the joints. In a pilot study presented at the American College of Rheumatology (ACR) Annual Meeting in Atlanta in November, investigators showed that DYV-700 provides meaningful and significant (p=0.004) pain reduction in minutes, not days, and reduced gout attack duration by nearly 50%. The treatment was safe and well tolerated. The initial clinical data along with encouraging evidence from pharmacokinetic, preclinical, and proof of concept studies from other drug candidates, show that Dyve’s technology has broad applicability and has the potential to help patients across multiple therapeutic areas.
About Dyve Biosciences

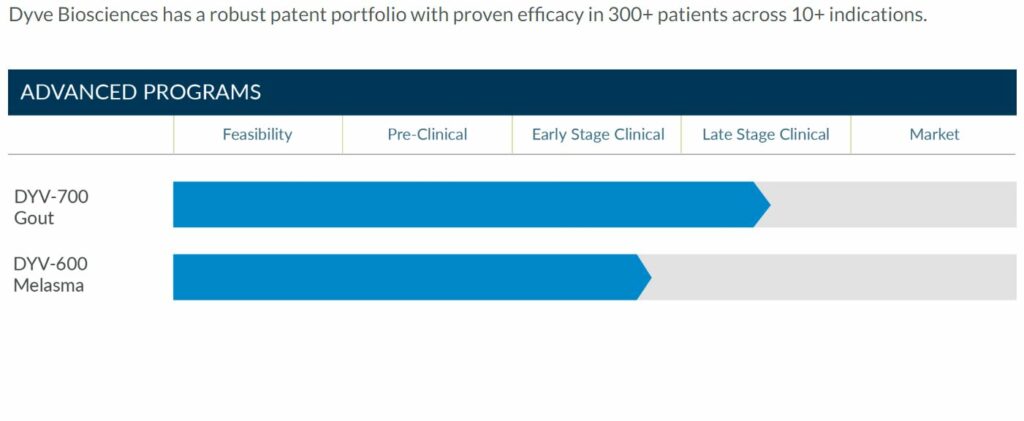
Dyve Biosciences has developed a breakthrough topical skin penetration technology. The innovative approach enables drugs to be transported from the surface of the skin into the blood with needle-like speed and pill-like efficiency. Dyve has advanced clinical programs in gout and melasma and a robust pre-clinical and clinical data set across a broad set of molecules. At Dyve, the focus is on robust science, compelling data, and delivering positive patient outcomes. For more information please visit dyvebio.com.
Contacts
Audra Friis
audra@pascalecommunications.com
917.519.9577