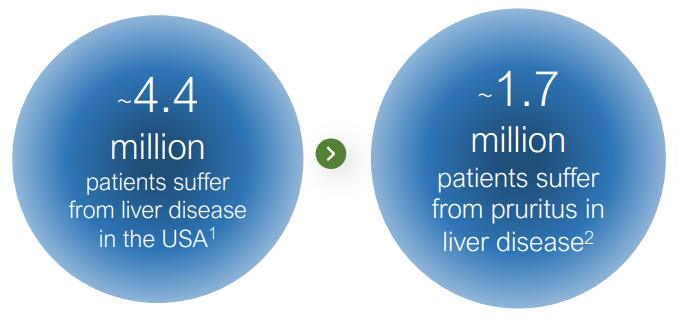
This is a story of ‘re-purposed’ FDA approved drug, from drug overdose, to a solution for unrelenting itching. If approved, it can provide a much needed relief from what is described as feeling like “bugs under the skin” to 1.7 million patients. Imagine an itch, you scratch it, and the itch doesn’t go away..
Phase I says it’s safe.
Investor presentation tonight in Boca Raton, FL with Randy Milby, who was CEO of Cormedix (CRMD) which grew from a $3 million market cap, to $277 million currently.
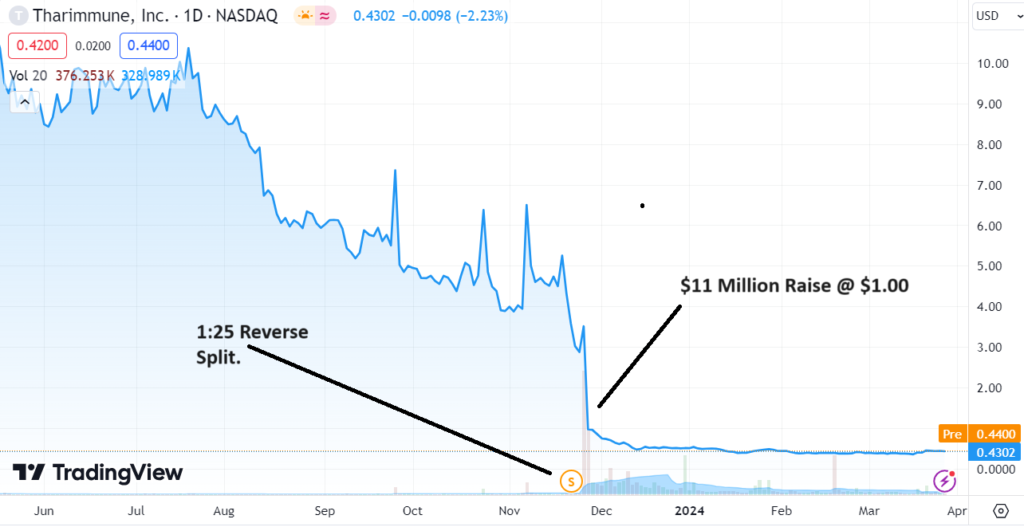
Number of common shares outstanding as of February 20, 2024 was 11,739,676. Wait, what, a $5 million market cap!
Tharimmune, Inc. (THAR) acquired the exclusive worldwide license for the clinical-stage asset, known to suppress chronic, debilitating pruritis or “uncontrollable itching” in PBC, a rare and orphan sized disease of the liver, with no known cure.
PBC is ‘primary biliary cholangitis‘ an autoimmune disease in which the bile ducts are inflamed and slowly destroyed. It previously was called primary biliary cirrhosis. Autoimmune means your body’s immune system is mistakenly attacking healthy cells and tissue.
When symptoms occur, they can include severe and unrelenting itching, fatigue, or yellowing of the skin.
TH104 is a proprietary transmucosal buccal film embedded with the active compound nalmefene onto a thin film which easily adheres inside of the mouth on the cheek and works in minutes.
Nalmefene (nalmefene hydrochloride nasal spray) was approved by the FDA in May 2023 to reverse opioid overdose. Nalmefene is an opioid receptor antagonist that is used to treat acute opioid overdose. The approved product is available by prescription, and is intended for use in health care and community settings.
Read more on Reverse Stock Split Big Gainers.
Tharimmune’s thin film provides key features making TH104 an ideal product candidate for multiple liver-related and other pruritogenic inflammatory conditions. The molecule has a dual mechanism of action affecting both the µ-opioid and kappa opioid receptors with emerging data showing inhibition of interleukin-17, a pro-inflammatory cytokine. These well-known opioid receptors when stimulated and/or inhibited by the body’s endogenous ligands have been shown to be involved in the body’s itch circuitry for certain conditions, including cholestatic or dysregulated bile acid-related liver conditions.
The company had a 1:25 reverse split in November 21st, 2023. They announced positive results from their Phase I study on February 23, 2024.
> The Phase 1 study demonstrated that TH104 had a comparable safety and tolerability profile to the FDA approved nalmefene reference intravenous formulation of the drug.
> There were no deaths, serious adverse events, or other significant adverse events reported during the entire study.
> Patients showed a 33.3% decline in 24-hour itch intensity when administered a single low-dose.
> Randy Milby stated “We are pleased with the completion of our Phase 1 study of TH104 and our expectations for a safe and tolerable transmucosal film delivery have been met.”
At the time of the news the Milby also stated “After the closing of an $11 million public offering which it believes is sufficient to extend its cash runway into early 2025 for clinical readouts of its lead program, TH104. The Company plans to advance both its clinical and non-clinical programs and announce an R&D Day in 2Q24 to update stakeholders and patients.”
SHORT TERM CHART

RECENT NEWS
Tharimmune Announces Positive Results in Phase I Clinical Trial
Tharimmune Announces Dosing of First Patient in Phase I
Tharimmune Announces Positive Topline Data
Tharimmune, Inc. Announces Closing of $11 Million Public Offering
VIDEO PRESENTATION
About Tharimmune
Tharimmune, Inc. is a clinical-stage biotechnology company developing a portfolio of therapeutic candidates for inflammation and immunology. The Company’s lead clinical-stage asset, TH104 is known to suppress chronic, debilitating pruritus or “uncontrollable itching” in PBC, a rare and orphan liver disease with no known cure. The Company’s early-stage immunology pipeline includes novel multi-specific antibodies targeting unique epitopes with novel mechanisms of action against well-known, validated targets in multiple solid tumors, including PD-1, HER2 and HER3. Tharimmune has a license agreement with OmniAb, Inc. to access the company’s antibody discovery technology platform against these and other specified targets. For more information please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding Tharimmune’s strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K/A for the year ended December 31, 2022 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. Subsequent events and developments may cause the Company’s views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
Investor Relations Contact
i[email protected]
www.tharimmune.com
SOURCE: Tharimmune, Inc.












