As is From $0.80 to $8.00..
One of our favorite Broker/Dealer Analysts thinks it is..
Positive news today from an old favorite Citius Pharma (CTXR), now looking better than it ever has (as in poised), following its third advancement with the FDA. Despite three major advancements in the past three months, the share price hasn’t moved.
The rubber band is really getting stretched.
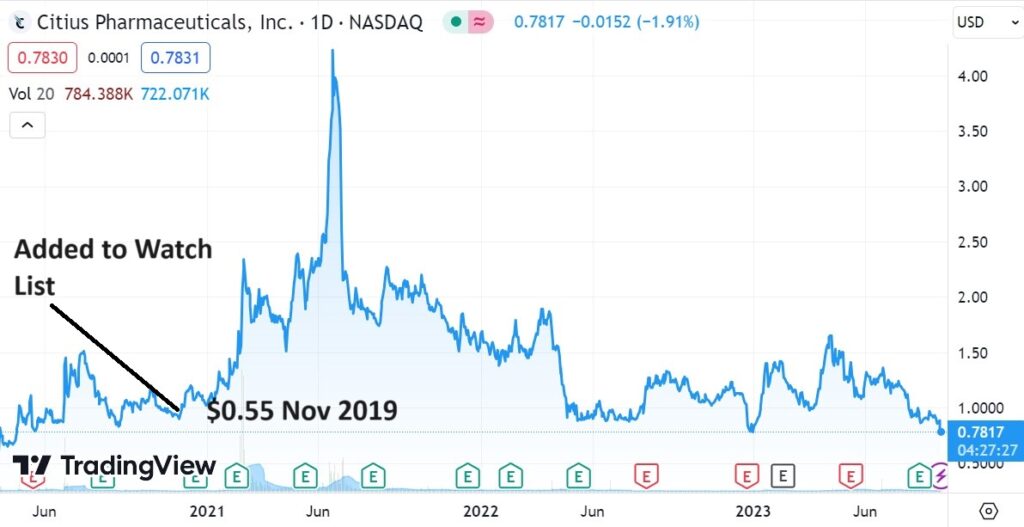
SHORT TERM CHART
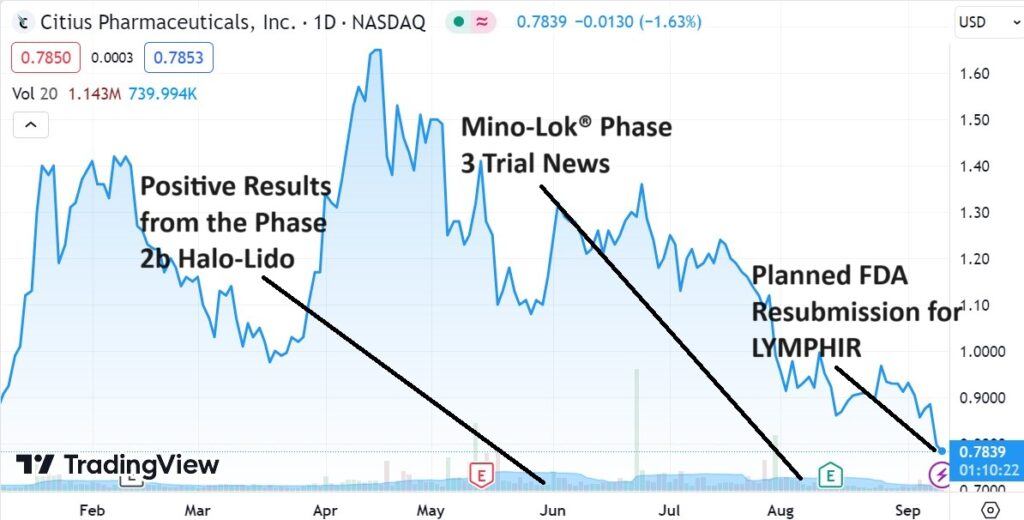
Three Shots on Goal
News is coming out fast and furious, which we expect to accelerate. Buckle up!
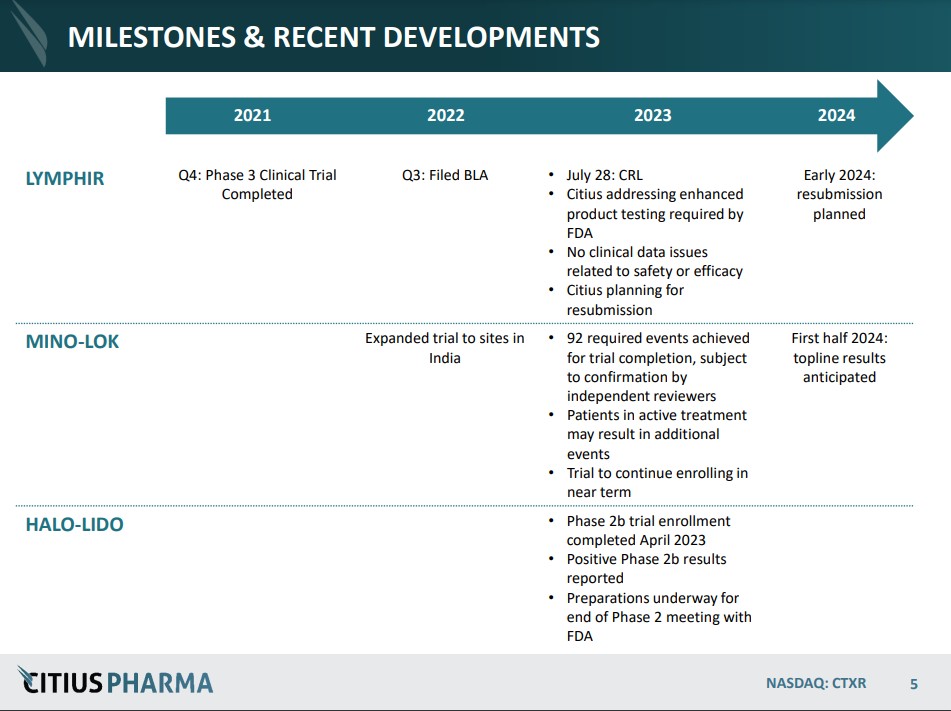
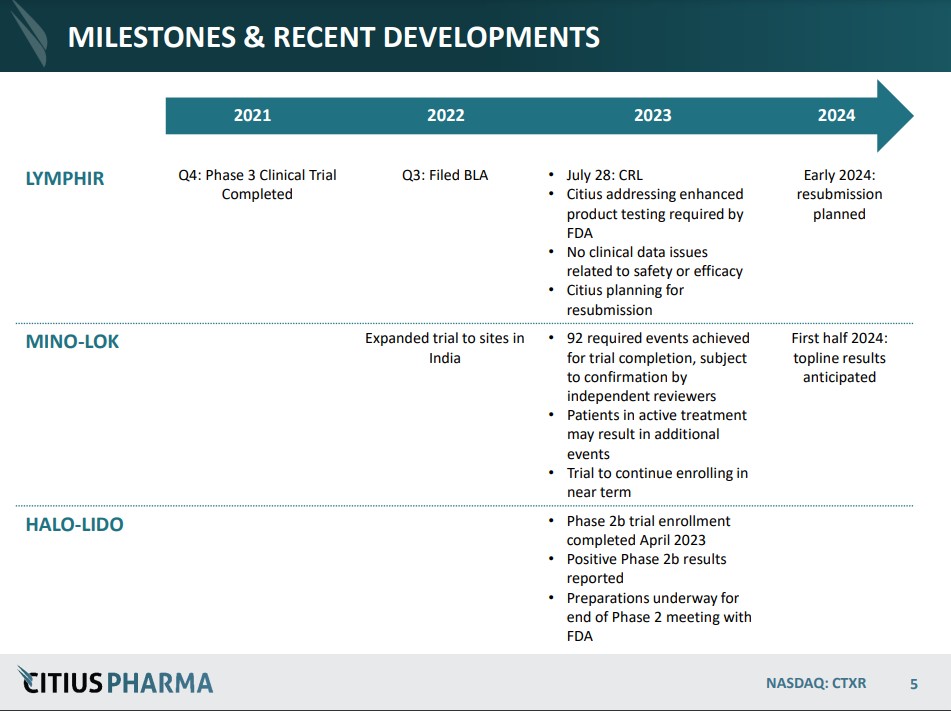
September 8th: Guidance from the FDA provides Citius with a path for completing resubmission of LYMPHIR (for non-Hodgkin lymphoma). No additional clinical efficacy or safety trials have been requested by FDA for the resubmission. Citius plans to complete the activities by the end of the year and file the resubmission in early 2024. Press Release.
August 10th: Pending confirmation from an adjudication committee of independent reviewers on Mino-Lok (for catheter-related blood stream infections), the Company believes all 92 events required to complete the trial have been achieved. Press Release.
June 20th: Announces positive results from the Company’s Phase 2b clinical study of Halo-Lido (meaningful reduction in symptom severity over lidocaine). Based on the positive clinical results utilizing the ‘Meaningful Change Threshold’ analysis. Citius plans to present this data at the end of Phase 2 meeting with the FDA. Their preference is to seek a development partner to further advance the treatment (Phase 3). The market for a product like this is estimated at $725 million.
Wall Street Coverage
One of our favorite small-cap analysts at brokerage firm Dawson James has an $8.00 price target. With regards to LYMPHIR they do not expect issues to require re-tooling and as such likely represent a type I (2-month delay). LYMPHIR and Mino–Lok report.
With regards to Halo-Lido the analyst noted a meaningful reduction in symptom severity, as reported by patients (42%), when compared to individual components alone. Halobetasol alone (29%) or patients treated with lidocaine alone (21%). Halo-Lido report.
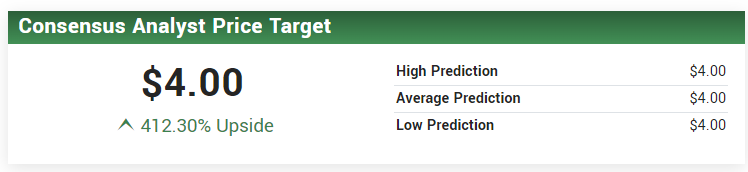
HC WAINWRIGHT CARRIES A $4.00 PRICE TARGET
Recent News
About Citius Pharmaceuticals, Inc.
Citius is a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products, with a focus on oncology, anti-infectives in adjunct cancer care, unique prescription products, and stem cell therapies. The Company’s diversified pipeline includes two late-stage product candidates, Mino-Lok®, an antibiotic lock solution for the treatment of patients with catheter-related bloodstream infections, which is currently enrolling patients in a Phase 3 Pivotal superiority trial, and I/ONTAK (E7777), a novel IL-2R immunotherapy for an initial indication in CTCL, for which a BLA is under review by the FDA. Mino-Lok® was granted Fast Track designation by the FDA. I/ONTAK has received orphan drug designation by the FDA for the treatment of CTCL and PTCL. At the end of March 2023 , Citius completed enrollment in its Phase 2b trial of CITI-002, a topical formulation for the relief of hemorrhoids. For more information, please visit www.citiuspharma.com .
Safe Harbor
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “plan,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: risks relating to the results of research and development activities, including those from existing and new pipeline assets, including CITI-002; the estimated markets for our product candidates and the acceptance thereof by any market; the ability of our product candidates to impact the quality of life of our target patient populations; our ability to commercialize our products if approved by the FDA; patent and intellectual property matters; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; uncertainties relating to preclinical and clinical testing; the early stage of products under development; our need for substantial additional funds; our dependence on third-party suppliers; our ability to procure cGMP commercial-scale supply; market and other conditions; our ability to attract, integrate, and retain key personnel; risks related to our growth strategy; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further impacted by Covid-19 and could be impacted by any future public health risks. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”) filings which are available on the SEC’s website at www.sec.gov , including in our Annual Report on Form 10-K for the year ended September 30, 2022 , filed with the SEC on December 22, 2022 and updated by our subsequent filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law. Past client, see published reports for disclosure and disclaimer details.
Investor Contact:
Ilanit Allen
ir@citiuspharma.com
908-967-6677 x113
Media Contact:
STiR-communications
Greg Salsburg
Greg@STiR-communications.com
#CTXR