Huge News: Gedeptin® Therapy Demonstrated Safety, Stabilization and Shrinkage of Treated Tumors.
GeoVax (GOVX) Completes Patient Enrollment for Head & Neck Cancer Drug, Gedeptin.®

David Dodd, chairman and CEO at GeoVax (GOVX), has seen a lot of action and fun during his 45 years in the biopharmaceuticals industry. He enjoyed numerous executive roles at Big Pharma’s including his start with Abbott in 1977, to both Bristol-Myers Squibb and later at Wyeth, until 1995.
After his career at big Pharma, his transformational skills working with startups such as Solvay Pharmaceutical and Serologics (SERO) became legendary, leading to his founding the highly regarded and well-known life sciences investment firm RiversEdge BioVentures. David knows the biotech market, like few others.
Now it appears he’s well along the path to scoring once again, by announcing yesterday that GeoVax has just completed patient enrollment, in one of their key drugs. With the completed enrollment, they now anticipate successfully completing the next phase in the FDA trial, in a time-period as short as nine-months away!
It should be noted, completing patient enrollment is never a given. Over the last 40 years we’ve witnessed dozens of companies fail to complete their patient enrollment goals. We’ve seen enrollments projected to be completed within a year, get dragged out for multi-years. And experienced biotech investors witnessed many trials fail to get completed altogether, during 2020 to 2022, due to third party events like the pandemic. The pandemic obliterated the patient base, willing to undergo a trail. No patient’s, no trial.
So, this is huge news folks. It’s ‘game-on’ news!
EXPERIENCE WITH STARTUPS
In 1995 Dodd left Wyeth Pharmaceuticals (it was later acquired by Pfizer (PFE) for $58 billion), to join Solvay Pharmaceuticals as CEO (from 1995 to 2000). During his 5 year tenure there Solvay’s enterprise value increased from $100 million to $2.5 billion, and ultimately to $6.2 billion in 2006. When it was acquired by Abbott Labs (ABT).
David Dodd Joins Solvay Pharma as CEO
Abbott Acquires Solvay
After Solvay, he became President of publicly traded Serologicals (SER0) during a management shake-up in 2000. The share price of SERO had fallen from $30 to $3.00 during 1999, the year before his arrival.
Within six years it rose to $31.55, with the market cap rising from $85 million to $1.4 billion – after it was acquired by Millipore. Cha-Ching! And all from the comfort near his hometown Atlanta, GA where his latest endeavor GeoVax, not so coincidentally, is also located.
By the time of the acquisition, SERO had grown to become the world’s leading provider of monoclonal antibodies for the blood typing industry. Serologicals employed approximately 1,000 people worldwide and reported revenues of $275 million. Millipore itself was acquired by Merck (MRK) in 2010 for $7.2 billion. Are we seeing a pattern here?
David Dodd Joins Serologicals
Millipore Acquires Serologicals
Total combined gains, $3.9 billion, Not bad for eleven years work, we think you’ll agree! We couldn’t possibly think of CEO more suited to reignite the clinical trial process and valuation of GeoVax, which is currently valued at a mere $10 million. Chump change.
Subscribe for our upcoming report on GeoVax.
Most recent GOVX S1-Filing, a mandatory read for investors conducting due diligence.
GeoVax Announces Gedeptin® Patient Enrollment Closure for Phase 1/2 Clinical Trial Among Advanced Head and Neck Cancer Patients.
Therapy Demonstrated Safety, Stabilization/Shrinkage of Treated Tumors
Expanded Development for Monotherapy and Combination Therapy Anticipated

ATLANTA, GA, Jan. 04, 2024 (GLOBE NEWSWIRE) — via NewMediaWire – GeoVax Labs, Inc. (Nasdaq: GOVX), a biotechnology company developing immunotherapies and vaccines against cancers and infectious diseases, today announced the closure of patient enrollment for the Phase 1/2 clinical study evaluating Gedeptin® in patients suffering from advanced head and neck cancer.
Kelly McKee, MD, MPH, GeoVax’s Chief Medical Officer, stated, “Completion of this trial will be a significant milestone in our Gedeptin clinical development program. Allowing time for the maximum number of cycles of Gedeptin therapy and patient follow-up, we expect to complete the study by the third quarter of this year. In the interim, we are in active discussions with advisors on protocol development in support of a follow-on Phase 2 or Phase 2/3 trial among patients with advanced head and neck cancer in whom current therapeutic options are suboptimal.
Our intent is to discuss this follow-on protocol with the FDA, in conjunction with a complete review of the results of the current trial, to ensure alignment with the regulator’s expectations. We expect that such discussions will include addressing the opportunity and basis for an expedited approval pathway.”
Dr. McKee continued, “Demonstrating the safety, tolerability, and stabilization or shrinkage of injected tumors in patients receiving multiple cycles of Gedeptin opens the door to advancing this promising therapeutic in additional patients with advanced head and neck cancer as well as in patients with other solid tumor types and at multiple points in their therapeutic journey.”
David Dodd, GeoVax’s Chairman and CEO, commented, “We believe that the successful completion of the current trial, in conjunction with earlier findings from the completed Phase 1 first-in-human trial and preclinical investigations, provide a sound rationale for proceeding with further Gedeptin investigations. These will include adjustments to the Gedeptin treatment regimen and combination with immune checkpoint inhibitors in advanced head and neck cancer as well as for additional cancerous and non-cancerous tumor indications. These advances represent a significant potential opportunity for GeoVax to improve the performance of immune checkpoint inhibitors* and/or introduce Gedeptin as a treatment option in patients with earlier-stage disease.”
About Gedeptin®
Gedeptin is a novel patented product/technology for the treatment of solid tumors through a gene therapy strategy known as Gene-Directed Enzyme Prodrug Therapy (GDEPT). In GDEPT, a vector is used to selectively transduce tumor cells with a nonhuman gene, which expresses an enzyme that can convert a nontoxic prodrug into a very toxic antitumor compound in situ.
The ongoing Phase 1/2 trial (ClinicalTrials.gov Identifier: NCT03754933) is evaluating the safety and efficacy of repeat cycles of Gedeptin therapy in patients with recurrent head and neck squamous cell carcinoma (HNSCC), with tumor(s) accessible for injection and no curable treatment options. The protocol entails up to five treatment cycles, each consisting of three intratumoral injections of Gedeptin over two days followed by infusion of a prodrug, fludarabine phosphate, once a day for three days. A completed Phase 1 dose-ranging study demonstrated that treating a tumor with a single cycle of Gedeptin, followed by fludarabine infusions, was well tolerated, with evidence of a reduction in tumor size in patients with solid tumors.
A previously reported interim data review demonstrated:
- No dose limiting toxicities or serious adverse events (SAEs) are definitively attributable to treatment. Additionally, no adverse events above grade 3 severity have been reported.
- Up to 5 cycles of Gedeptin treatment have been administered without limiting sequelae. Intratumoral expression of the PNP transgene by RT-PCR has been established in treated tumors studied to date.
- Impairment of tumor growth (i.e., “stable disease” using RECIST 1.1 evaluation criteria) in targeted lesions was seen in 5 of 7 patients; tumor response assessment in one patient remains under study.
The current study is being funded in part by the FDA pursuant to its Orphan Products Clinical Trials Grants Program. The FDA has also granted Gedeptin orphan drug status for the intratumoral treatment of anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of mouth, salivary gland, and other oral cavities.
*List and Description of FDA Approved Immune Checkpoint Inhibitors
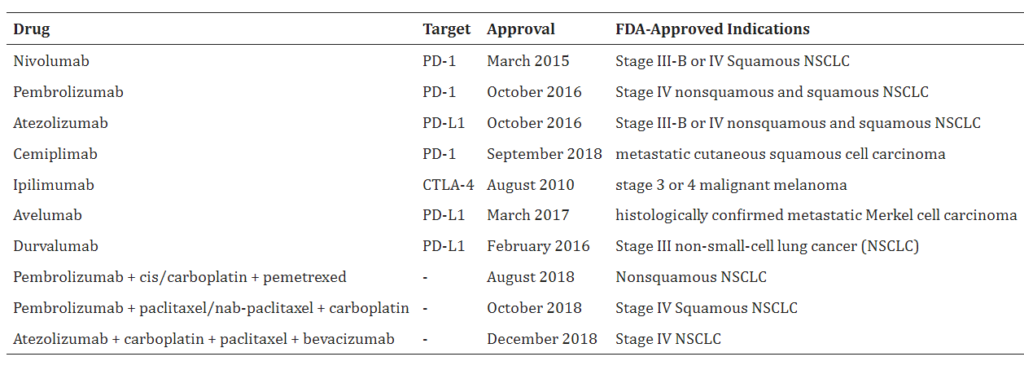
(in our upcoming report will list the names of the Companies that own these drugs)
About GeoVax
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing novel therapies and vaccines for solid tumor cancers and many of the world’s most threatening infectious diseases. The company’s lead program in oncology is a novel oncolytic solid tumor gene-directed therapy, Gedeptin®, presently in a multicenter Phase 1/2 clinical trial for advanced head and neck cancers. GeoVax’s lead infectious disease candidate is GEO-CM04S1, a next-generation COVID-19 vaccine targeting high-risk immunocompromised patient populations. Currently in three Phase 2 clinical trials, GEO-CM04S1 is being evaluated as a primary vaccine for immunocompromised patients such as those suffering from hematologic cancers and other patient populations for whom the current authorized COVID-19 vaccines are insufficient, and as a booster vaccine in patients with chronic lymphocytic leukemia (CLL). In addition, GEO-CM04S1 is in a Phase 2 clinical trial evaluating the vaccine as a more robust, durable COVID-19 booster among healthy patients who previously received the mRNA vaccines. GeoVax has a leadership team who have driven significant value creation across multiple life science companies over the past several decades. For more information, visit our website: www.geovax.com.
Forward-Looking Statements
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.
Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise. GOVX is a client of Institutional Analyst which receives a monthly retainer of five-thousand dollars for ongoing progress reporting and news coverage.
#GOVX












