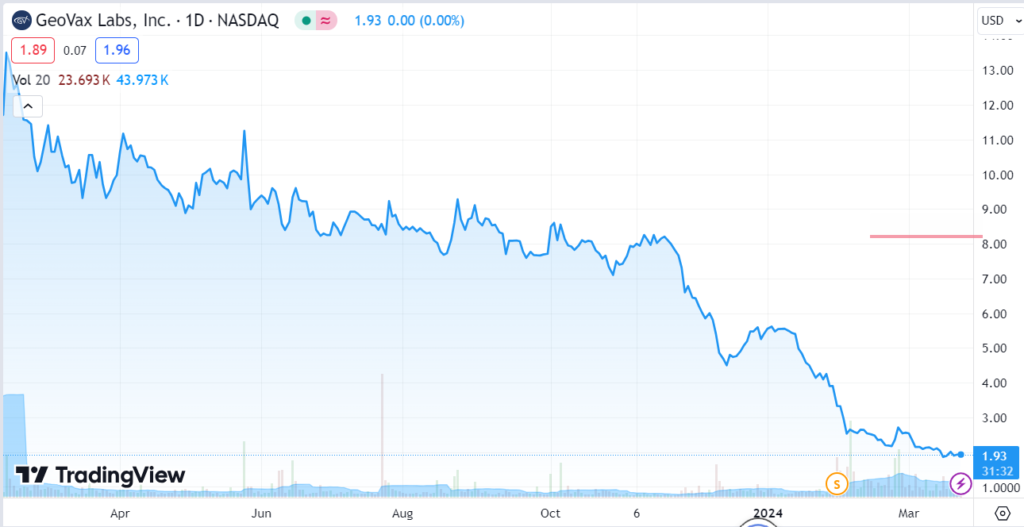
GeoVax (GOVX) intends to become the first U.S.-based supplier of the MVA vaccine to prevent Mpox and smallpox.
The World Health Organization just updated global monkeypox trends. The Lancet has stated “China still faces the risk of an mpox outbreak. Furthermore, as a country with a large population, the global spread of mpox could be exacerbated if local transmission is not controlled immediately.” They would be a good candidate for stockpiling mpox vaccines, in our opinion.
GeoVax intends to become the first U.S.-based supplier of the MVA vaccine to prevent Mpox and smallpox.
In response to the global need to address the continued emerging threat from Mpox (monkeypox), GeoVax (GOVX) previously announced having secured rights from the National Institutes of Health (NIH) covering preclinical, clinical and commercial uses of the NIH-MVA as a vaccine against Mpox or smallpox. The Company is currently pursuing different regulatory pathways toward achievement of an expedited approval and intends to become the first U.S.-based supplier of the MVA vaccine to prevent Mpox and smallpox.
GeoVax Achieves Milestone in Transition to Commercially Validated Manufacturing System
LANCET AND CHINA
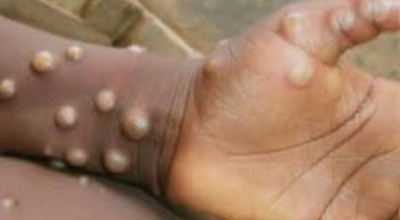
As an infectious disease caused by the monkeypox virus, mpox (formerly known as monkeypox) has gained global attention ever since several patients with mpox were identified in May, 2022, in various countries without a history of sustained community transmission.
On May 11, 2023, the mpox outbreak was no longer regarded as a public health emergency of international concern; however, in China, the number of patients with mpox has substantially increased since February, 2023. According to the global situation update from WHO, mpox cases are continuing to increase, indicating that China still faces the risk of an mpox outbreak. Furthermore, as a country with a large population, the global spread of mpox could be exacerbated if local transmission is not controlled immediately.
According to a 2023 report, investigating the willingness of 521 Chinese participants to receive a vaccine for mpox, most (398 [76·4%]) were willing to accept a vaccination, supporting the rationality of expanding the coverage of vaccines in people with a heightened risk of infection.
Therefore, the targeted vaccination of populations after a risk assessment for mpox infection based on the results of contact tracing could be more economical and effective at containing mpox.
Full report: Prevention of a potential mpox outbreak in China
WHO:
The mpox long-term risk was assessed as follows:
For the general population in countries newly affected in 2022-23 or not affected mpox risk is assessed as low.
For the general population in countries with historical mpox transmission and their neighboring countries mpox risk is assessed as moderate.
The overall global risk for men who have sex with men and sex workers is assessed as moderate.
This report should be considered in the context of other WHO information products associated with the 2022-23 mpox outbreak, and mpox in general. Links to these products can be found at the end of the report.
The monthly situation report provides a comprehensive update of the mpox situation and response activities across a variety of domains such as epidemiology, clinical management and communications;
This global epidemiological report provides in-depth epidemiological information about the mpox situation, based primarily on case report forms provided by Member States to WHO under Article 6 of the International Health Regulations (IHR 2005), and the Standing Recommendations for mpox.
Forward-Looking Statements This post contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control. Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law. GOVX is a client of Institutional Analyst, publisher of the Biotech Stock Review, which receives a monthly retainer of five-thousand dollars for ongoing progress reporting and news coverage.
GeoVax Labs, Inc. is followed by the analysts listed above. Please note that any opinions, estimates or forecasts regarding GeoVax Labs, Inc.’s performance made by these analysts (and us) are theirs alone and do not represent opinions, forecasts or predictions of GeoVax Labs, Inc. or its management. GeoVax Labs, Inc. does not by its reference below or distribution imply its endorsement of or concurrence with such information, conclusions or recommendations.












