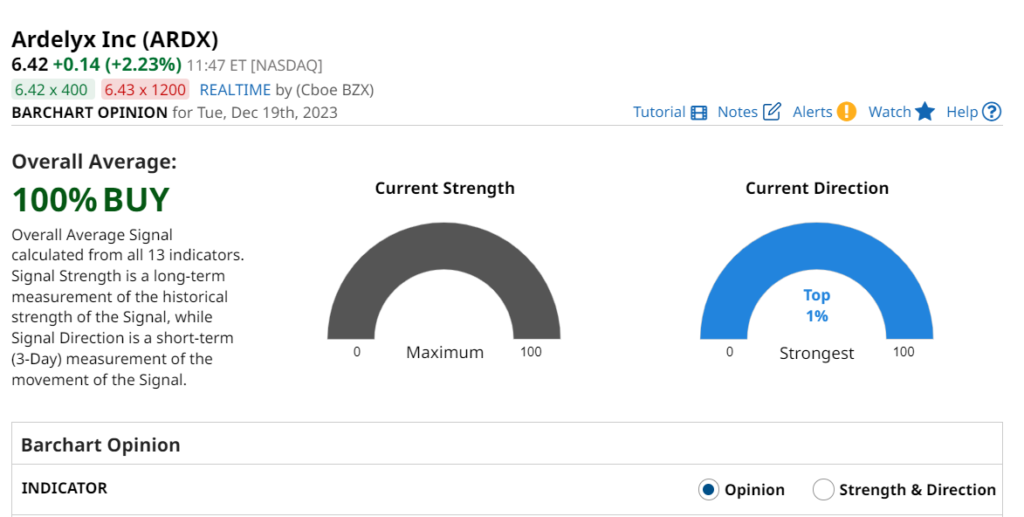
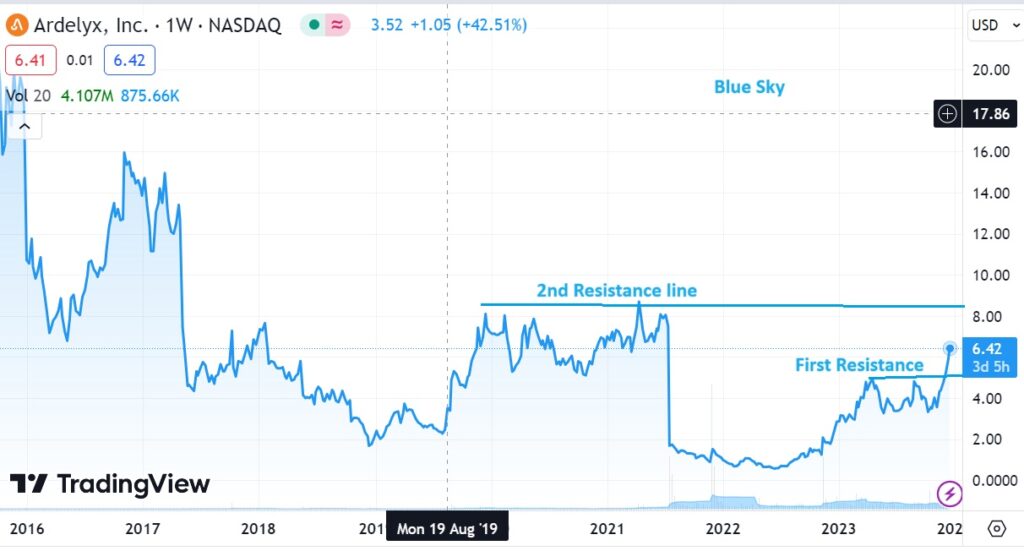
Were not traders and we don’t do technical analysis, but we could see this coming from a mile away. Next stop $9.00 and then we’re free to move to our mental target in the $20’s. At $15.70 we’ll have our 14th ten-bagger.
As for now, we’re up 308% and that ain’t bad..
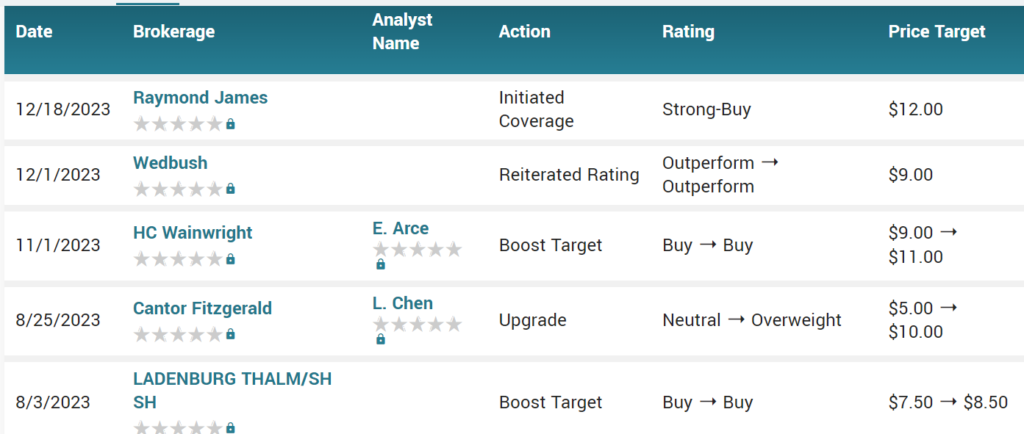
The highest target on the street is $12, from Raymond James yesterday.
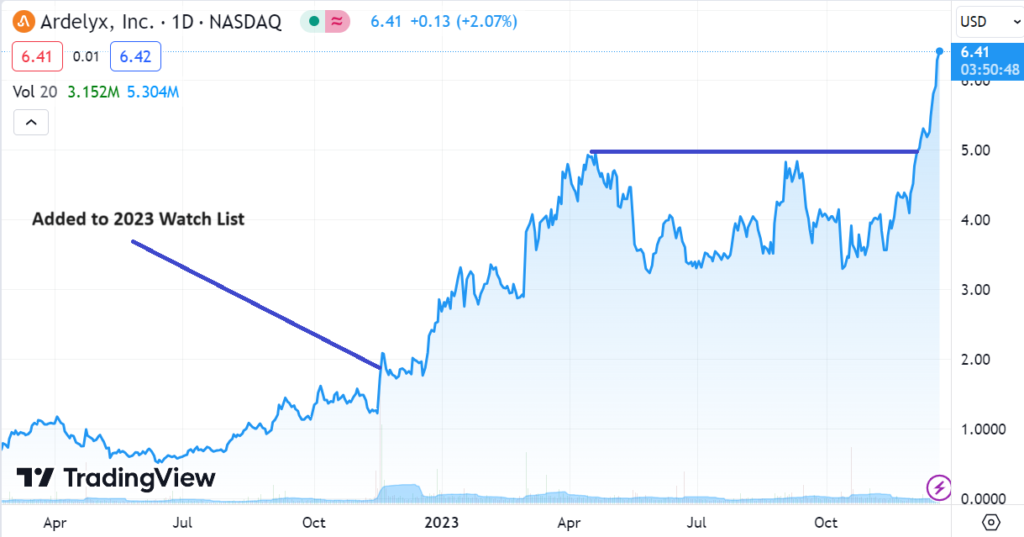
TECHNICAL INDICATORS ALL TURN GREEN
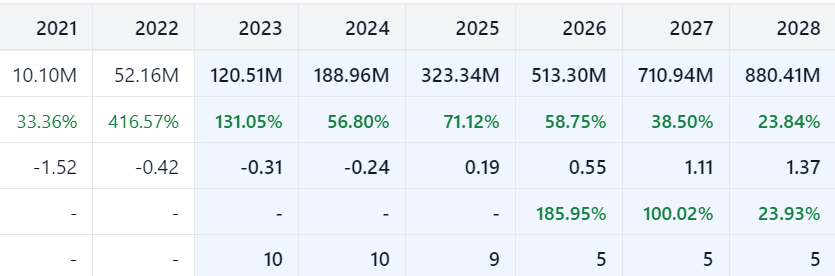
About Ardelyx, Inc.
Ardelyx was founded with a mission to discover, develop and commercialize innovative, first-in-class medicines that meet significant unmet medical needs. Ardelyx has two commercial products approved in the United States, IBSRELA® (tenapanor) and XPHOZAH® (tenapanor), as well as early-stage pipeline candidates. Ardelyx has agreements for the development and commercialization of tenapanor outside of the U.S. Kyowa Kirin has received approval for PHOZEVEL® (tenapanor) for hyperphosphatemia in Japan. A New Drug Application for tenapanor for hyperphosphatemia has been submitted in China with Fosun Pharma. Knight Therapeutics commercializes IBSRELA in Canada. For more information, please visit https://ardelyx.com/ and connect with us on X (formerly known as Twitter), LinkedIn and Facebook.












