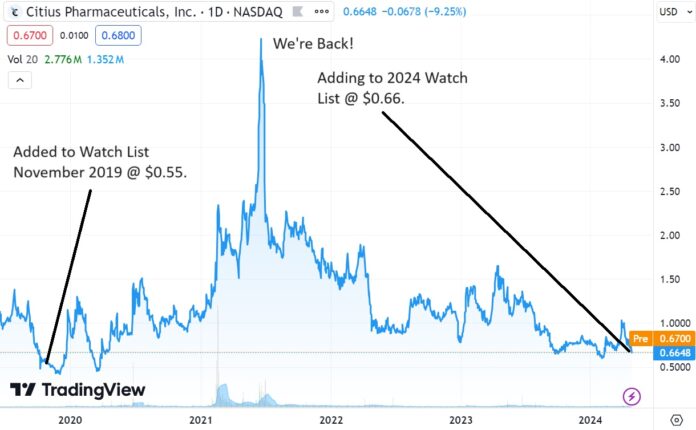
Three Years Since we Last Added Citius (CTXR) to the Watch List, and it Looks Better Than Ever!
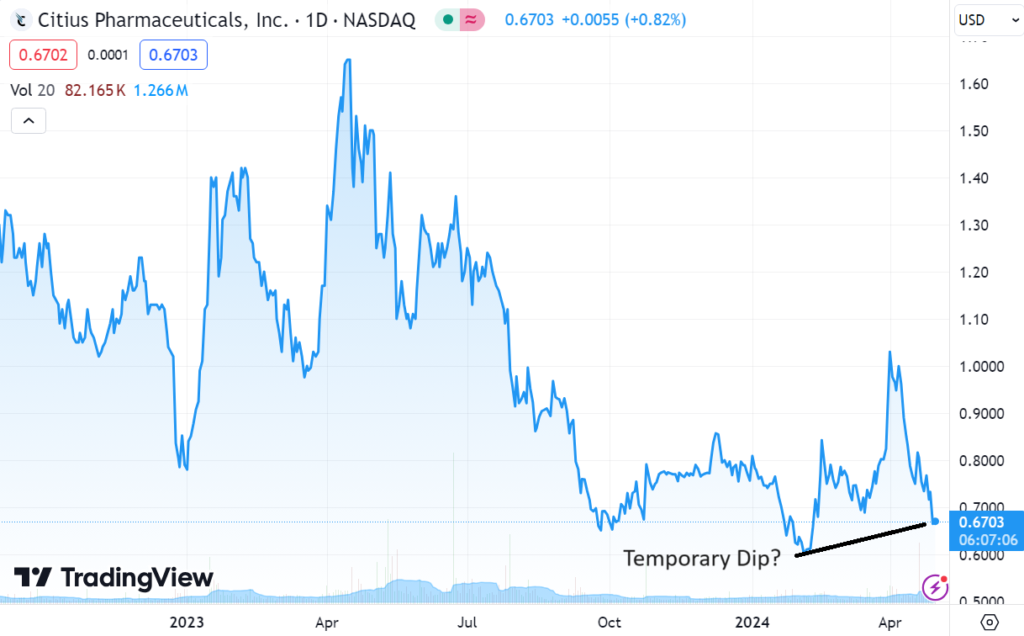
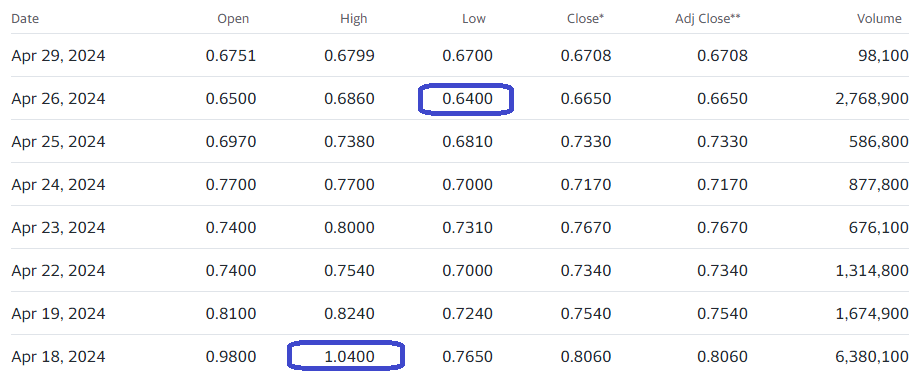
We’re adding Citius Pharma to the Watch List, on the heels of a small $15 million raise. Details to follow later in the week. Short story is when most biotech companies do an interim raise when (or shortly after) releasing interim data, the shares often hit a temporary air pocket.
We are not traders, so we are not trading for a short-term bounce (like $0.66 back to $1.00). We are long-term and the way we look at it, these dips can be an excellent opportunity for long-term investors to acquire a significant stake at a temporarily depressed price, without moving the stock up.
In contrast to most investors who have dilution-phobia, we can list dozens of instances where it was the best time to initiate a position. Just saying..
There is a lot of expected news coming up in the near-term, which we will report on in the coming week.
We think it’s a good time to get locked and loaded.
Subscribe for upcoming Citius (CTXR) report.
Killer, 44-Page Report on GeoVax (GOVX) $1.50.
Citius Pharmaceuticals Announces $15 Million Registered Direct Offering
CRANFORD, N.J., April 26, 2024 /PRNewswire/ — Citius Pharmaceuticals Inc. (Nasdaq: CTXR) (“Citius” or the “Company”), a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products, today announced that it has entered into definitive agreements for the purchase of an aggregate of 21,428,574 shares of its common stock and accompanying warrants to purchase up to an aggregate of 21,428,574 shares of its common stock, at a purchase price of $0.70 per share and accompanying warrant in a registered direct offering. The warrants will have an exercise price of $0.75 per share, will be exercisable six months from the date of issuance, and will expire five years from the initial exercise date. The closing of the offering is expected to occur on or about April 30, 2024, subject to the satisfaction of customary closing conditions.
H.C. Wainwright & Co. is acting as the exclusive placement agent for the offering.
The aggregate gross proceeds to the Company from the offering are expected to be approximately $15 million, before deducting the placement agent fees and other offering expenses payable by the Company. Citius currently intends to use the net proceeds from the offering for general corporate purposes, including pre-clinical and clinical development of our product candidates and working capital and capital expenditures.
The securities described above are being offered pursuant to a “shelf” registration statement (File No. 333-277319) filed with the Securities and Exchange Commission (“SEC”) on February 23, 2024 and declared effective on March 1, 2024. The offering is being made only by means of a prospectus, including a prospectus supplement, forming a part of the effective registration statement. The prospectus supplement and the accompanying prospectus relating to the securities being offered will be filed with the SEC and be available at the SEC’s website at www.sec.gov. Electronic copies of the prospectus supplement and the accompanying prospectus relating to the securities being offered may also be obtained, when available, by contacting H.C. Wainwright & Co., LLC at 430 Park Avenue, 3rd Floor, New York, NY 10022, by telephone at (212) 856-5711 or e-mail at [email protected].
This press release shall not constitute an offer to sell or the solicitation of an offer to buy any of the securities described herein, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or jurisdiction.
About Citius Pharmaceuticals, Inc.
Citius is a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products. The Company’s diversified pipeline includes two late-stage product candidates. The Biologics License Application for LYMPHIRTM, a novel IL-2R immunotherapy for an initial indication in cutaneous T-cell lymphoma, is currently under review by the FDA with August 13, 2024 assigned as the PDUFA target action date. Citius previously announced plans to form Citius Oncology, a standalone publicly traded company with LYMPHIR as its primary asset. LYMPHIR received orphan drug designation by the FDA for the treatment of CTCL and PTCL. In addition, Citius completed enrollment in its Phase 2b trial of CITI-002 (Halo-Lido), a topical formulation for the relief of hemorrhoids. For more information, please visit www.citiuspharma.com.
Forward Looking Statements
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “plan,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price, and includes all statements related to the completion of the offering, the satisfaction of customary closing conditions related to the offering and the intended use of net proceeds from the offering. Factors that could cause actual results to differ materially from those currently anticipated are: risks related to the closing of the offering; risks relating to the results of research and development activities, including those from existing and new pipeline assets; uncertainties relating to preclinical and clinical testing; the early stage of products under development; our need for substantial additional funds; our ability to commercialize our products if approved by the FDA; our dependence on third-party suppliers; our ability to procure cGMP commercial-scale supply; the estimated markets for our product candidates and the acceptance thereof by any market; the ability of our product candidates to impact the quality of life of our target patient populations; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; market and other conditions; our ability to attract, integrate, and retain key personnel; risks related to our growth strategy; patent and intellectual property matters; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further impacted by Covid-19 and could be impacted by any future public health risks. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our SEC filings which are available on the SEC’s website at www.sec.gov, including in our Annual Report on Form 10-K for the year ended September 30, 2023, filed with the SEC on December 29, 2023, and updated by our subsequent filings with the SEC. These forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law. Past news coverage and progress reporting client, see previous reports for disclaimer and disclosure details.
Investor Contact:
Ilanit Allen
[email protected]
908-967-6677 x113
Media Contact:
STiR-communications
Greg Salsburg
[email protected]