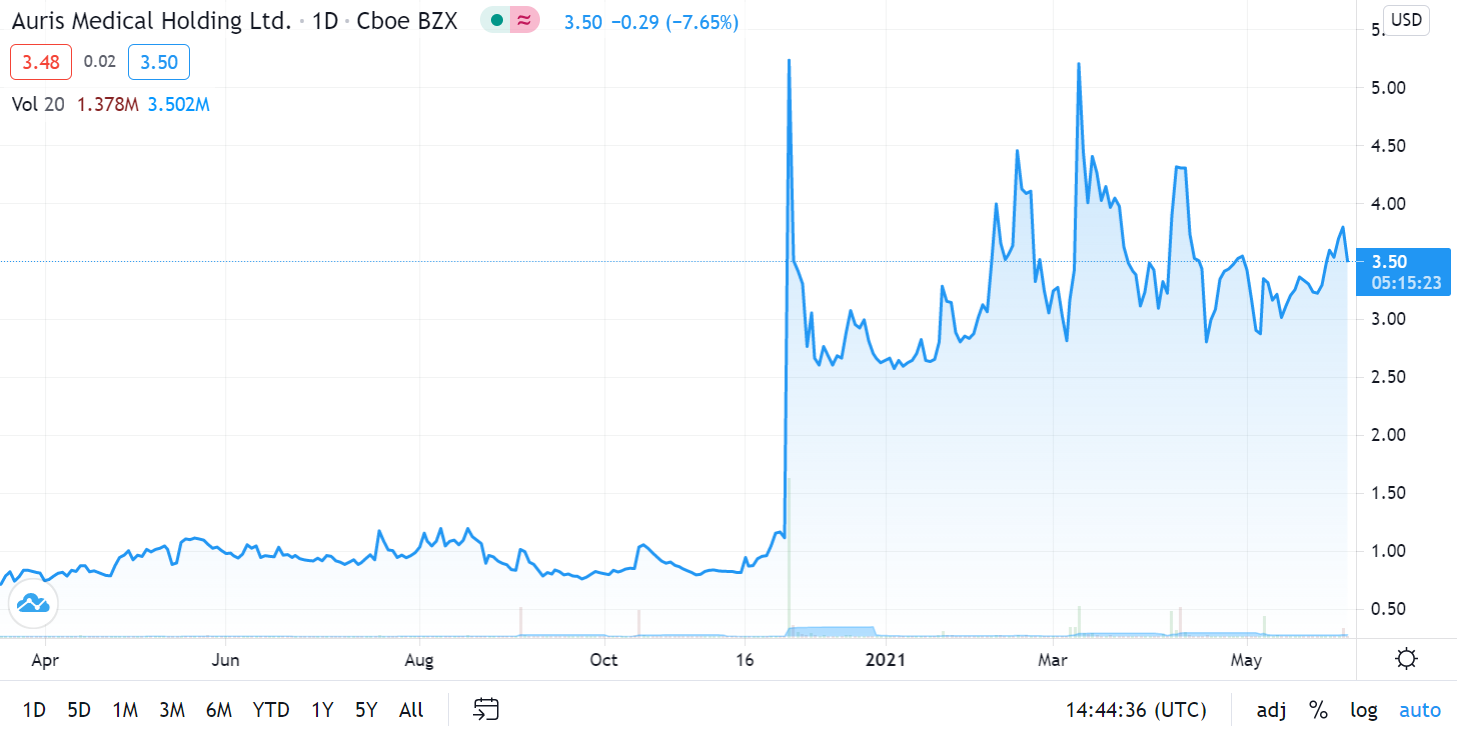
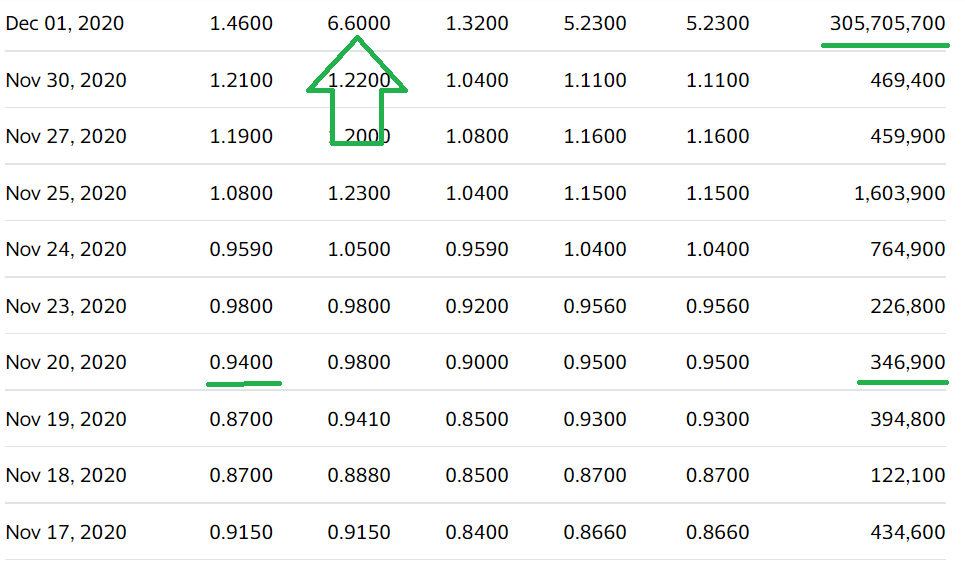
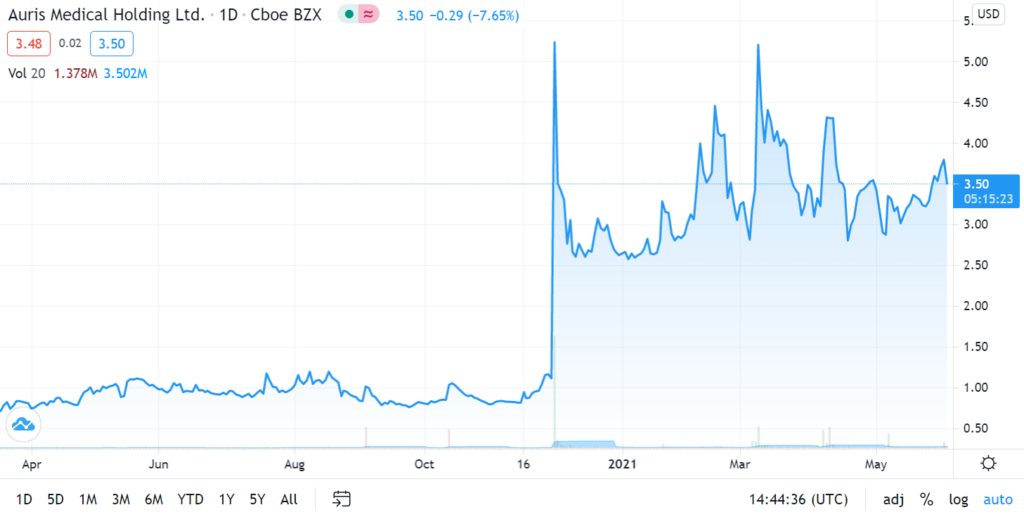
Auris Medical soared 590% in one Week on Covid Related News on December 1st, 2020.
While we were waiting for a further pullback, yesterday’s merger news suggests now may be an opportune time to establish a position, so we are adding it to the Watch List. In addition, news related to Bentrio their Covid related product – an allergan blocking nasal spray – is expected in third quarter 2021.
We’ll have more details next week. It traded as high as $4.58 on 15 million shares yesterday, up from 515,000 shares – to we are time and price stamping our interest here for now. Here are the two previous significant press releases:
Auris Medical soars 10% on acquisition of Trasir Therapeutics and plans for strategic repositioning
June 3rd, 2021. Auris Medical Holding (EARS) jumps 10% premarket after acquiring privately-held Trasir Therapeutics, based in Tampa, FL. The purchase price comprises 0.77M common shares of the acquiring Company, the assumption of certain selling shareholders’ cash outlays as well as a future share-based payment contingent on reaching a specific development milestone. The transaction is the starting point for a strategic repositioning under which the Company intends to focus on the development of RNA therapeutics while in the medium term aiming to spin off or divest its existing assets in neurotology, rhinology, and allergology. Trasir’s innovative peptide-based OligoPhore platform is designed to enable extrahepatic delivery of oligonucleotides. To reflect the repositioning, the Board of Directors of EARS intends to call an extraordinary general meeting of shareholders to propose its corporate name change to Altamira Therapeutics. Upon approval, the Company’s shares will start trading under the new ticker symbol “CYTO” instead of “EARS”.
Auris Medical soars after AM-301 shows encouraging preclinical action against SARS-CoV-2 infection.
December 1st, 2020. Auris Medical (EARS + 63.1%) has announced efficacy data from testing AM-301 in vitro, a drug-free nasal spray for protection against airborne pathogens and allergens. AM-301 was tested for its capability to prevent or mitigate SARS-CoV-2 infection of nasal epithelial cells. In saline-treated control cultures, Sars-CoV-2 replicated efficiently, resulting in a rapid increase in viral titer. In contrast, daily treatment with AM-301, beginning right before inoculation, showed effective protection against viral infection.48 hours post-infection, average virus titers were 90.0% lower than those observed in controls. 72 hours and 96 hours post-infection, average virus titers were 99.2 and 99.4% lower, respectively. The company says its looks forward to taking AM-301 through additional tests and advancing the program towards the submission of regulatory applications in 2021.
These press releases may contain statements that constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are statements other than historical facts and may include statements that address future operating, financial or business performance or Auris Medical’s strategies or expectations. In some cases, you can identify these statements by forward-looking words such as “may”, “might”, “will”, “should”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “predicts”, “projects”, “potential”, “outlook” or “continue”, or the negative of these terms or other comparable terminology. Forward-looking statements are based on management’s current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include, but are not limited to, the approval and timing of commercialization of AM-301, Auris Medical’s need for and ability to raise substantial additional funding to continue the development of its product candidates, the timing and conduct of clinical trials of Auris Medical’s product candidates, the clinical utility of Auris Medical’s product candidates, the timing or likelihood of regulatory filings and approvals, Auris Medical’s intellectual property position and Auris Medical’s financial position, including the impact of any future acquisitions, dispositions, partnerships, license transactions or changes to Auris Medical’s capital structure, including future securities offerings. These risks and uncertainties also include, but are not limited to, those described under the caption “Risk Factors” in Auris Medical’s Annual Report on Form 20-F for the year ended December 31, 2020, and in Auris Medical’s other filings with the SEC, which are available free of charge on the Securities Exchange Commission’s website at: www.sec.gov. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those indicated. All forward-looking statements and all subsequent written and oral forward-looking statements attributable to Auris Medical or to persons acting on behalf of Auris Medical are expressly qualified in their entirety by reference to these risks and uncertainties. You should not place undue reliance on forward-looking statements. Forward-looking statements speak only as of the date they are made, and Auris Medical does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law.
#altamira