Can a Swash Buckling Wall Street Analyst Lose Weight and get his Blood Sugar Down From Near 200 to Under 125?
First to be clear, this is not a clinical trial. This is a Wall Street Biotech analyst who wants to get in better shape trial. So let’s call it a ‘personal’ non-clinical trial.
The trial is conducted by the patient (personal trial or PNCT) and the PNCT data will be monitored and recorded by the Blood Sugar Institute.
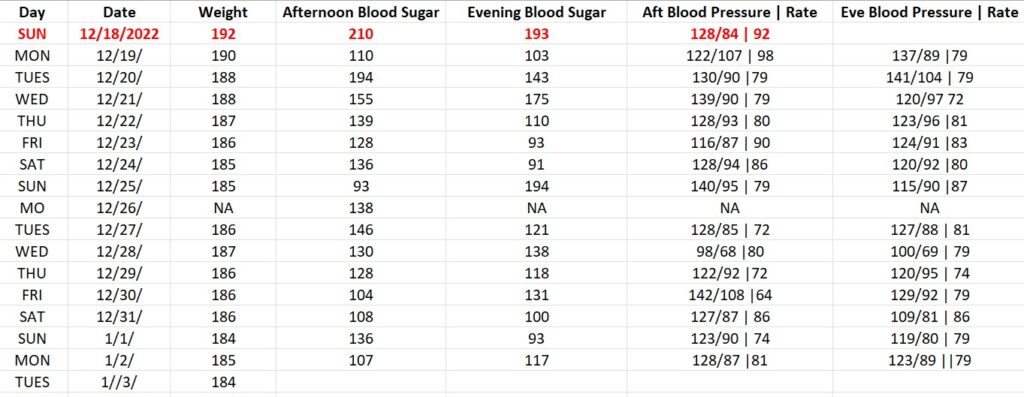
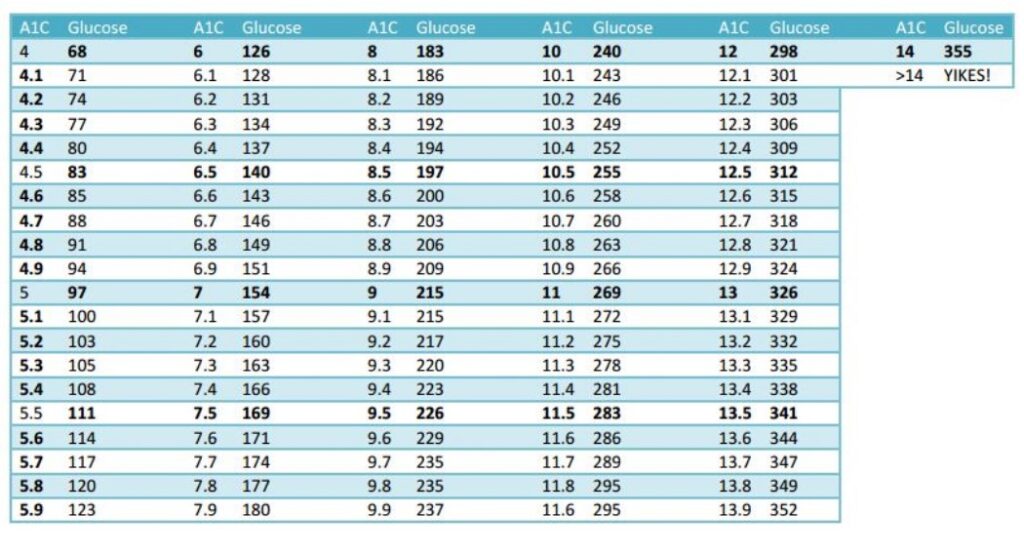
The trial endpoint goal for the PNCT are lowering his daily blood sugar from 193 to under 125 and lowering his A1C from 9 to under 6. The secondary endpoint is losing weight, from 192 pounds to a goal of 175.
On a daily basis we will be monitoring his blood sugar, blood pressure, and weight. We’ll use readily available monitoring tools, that anyone can buy at their local Walmart.
We’ll include daily notations on diet, drinking and exercise.
On a monthly basis we’ll do a full blood panel including cholesterol and triglycerides and 40 other important markers via Lab Testing API and share the results.

We’ll post both his daily and monthly progress below, for quick review.
To conduct the study he will be using a combination of a recently approved and revolutionary new drug from Eli Lilly & Company (LLY) called ‘Mounjaro‘ (or tirzepaitide) and a variety of OTC available supplements.
The combination of supplements include a revolutionary new nano-encapsuled BioSoluble curcummin, that has shown some impressive data related to blood sugar. Protein and fiber drinks, and a calcium bentonite clay that helps promote detoxification – which is good during periods of extensive weight loss.
The trial will last three months, at which time a decision will be made to continue Mounjaro, or to wean off it and rely solely on supplements, diet and exercise.
Of course, diet and exercise will play an important, if not the most important role in long term success.
And we begin!
BLOOD GLUCOSE REDUCTION TABLE | DAILY RESULTS
WE CAN DO THIS!

BLOOD SUGAR TRIAL | BLOG (coming soon)
NEWS
Mounjaro
Curcumin
The National Library of Medicine (NLM) which is the world’s largest medical library, founded in 1836, and operated as an institute within the National Institute of Health. recently conducted an extensive review of Curcumin in 2020.
National Library of Medicine Curcumin Review
DAILY BLOOD SUGAR TO A1C CONVERSION TABLE
MOUNJARO SAVINGS CARD

DISCLAIMER
The standard disclaimer is that the statements above (and in future blogs) have not been evaluated by the Food and Drug Administration. Neither the products used in the PNCT, nor is anything on this website, intended to diagnose, treat, cure, or prevent any disease. All true.
People taking Mounjaro, have lost up to 25 pounds. However Mounjaro is not a weight loss drug. It may be prescribed by Physicians for patients with Type 2 diabetes, who have been diagnosed with Type 2 diabetes.
Additional disclaimer. This website is not a guide on ‘how to’ lose weight, or reduce ones blood sugar levels. It is a personal record of events of one individuals efforts, to lose weight and reduce his blood sugar. Do not attempt to follow his steps, his unconventional diet and certainly not his drinking practices and habits. Doing so could cause serious harm.
This is a ‘how he’ lost weight and/or reduced his blood sugar levels guide, shared with friends and family. ‘How he,’ assuming he loses weight and/or reduces his blood sugar level, by the end of the trial. Which is by no means assured.
Financial disclaimer, we may receive compensation from the manufacturers of products used or mentioned in the Blood Sugar Trials, and/or from service providers mentioned on the website, via advertising or via affiliate fee based arrangements now and/or in the future.
Safety disclaimer. The most common side effects of Mounjaro include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and stomach (abdominal) pain. These are not all the possible side effects of Mounjaro. Many more serious complications can be found here on the Eli Lilly website under: Warnings
This website provides some basic information about Mounjaro but does not include all information known about this medicine. For users of Mounjara read the information that comes with the prescription each time the prescription is filled. Information on this website does not take the place of talking with a healthcare provider. Be sure to talk to a healthcare provider about Mounjaro and how to take it. A healthcare provider is the best person to help decide if Mounjaro is even right for you.
Mounjaro® and its delivery device base are registered trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
ABOUT THIRD PARTY LINKS ON OUR SITE
The Blood Sugar Trials (BST) website offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the BST website and will be redirected to another site. These sites are not under the control of BST and it is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information. Also, please be aware that the security and privacy policies on these sites may be different than BST policies, so please read third party privacy and security policies closely. If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.




