Two News Reports Related to Revives Upcoming Microneedle Patch Technology
While we are still laser focused on Revive having a successful meeting with the FDA on Bucillamine, they continue to advance a delivery technology with a goal of human trials.
The nice thing about Microneedle Patch’s are that they have the potential for better controlled release (over oral pills), which could potentially lead to ‘self-dosing’ at home, with built in safety features such as anti-tampering and anti-abuse features.
Microneedles are considered a promising way to achieve systemic effects by transdermal delivery of drugs, including psychedelics, and circumventing absorption and first-pass barriers typical for oral delivery.
As a delivery system, Revive will seek to determine which various indications MDMA has best promise with the technology, including depression, anxiety, abuse disorders (i.e. eating, alcohol and drug use), and post-traumatic stress disorder (“PTSD”). News related to MDMA’s actual effectiveness and the FDA’s decision for potential approval of MDMA-assisted therapy for PTSD is expected in 2024 via MAPS trials.
MAPS has completed two confirmatory Phase 3 trials of MDMA-assisted therapy for PTSD to potentially support its new drug application to be filed with the FDA.
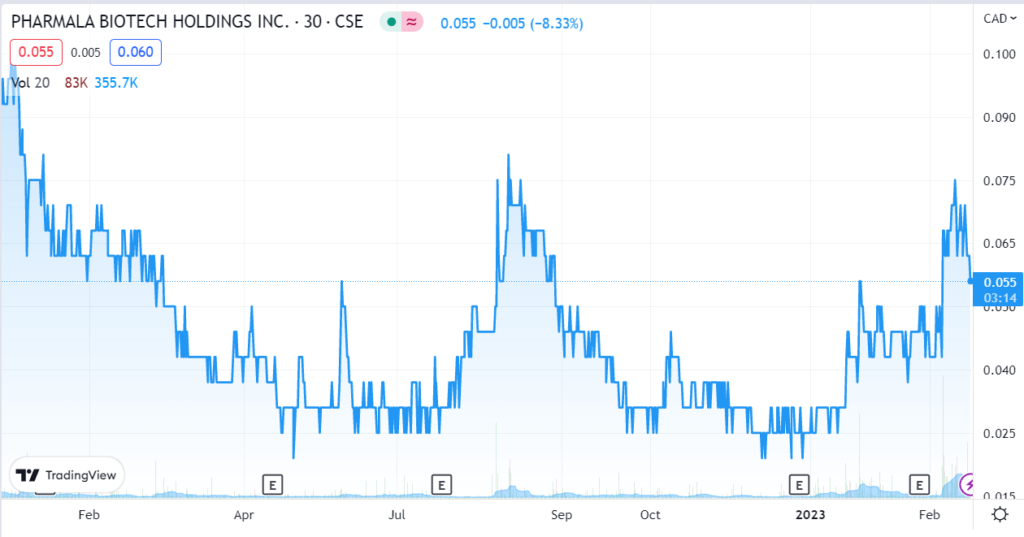
February 6th. PharmAla Biotech Holdings Inc. (MDMA) is pleased to announce that it has signed on to act as exclusive supplier of both GMP LaNeo MDMA and Engineering MDMA to Revive Therapeutics. Press Release.
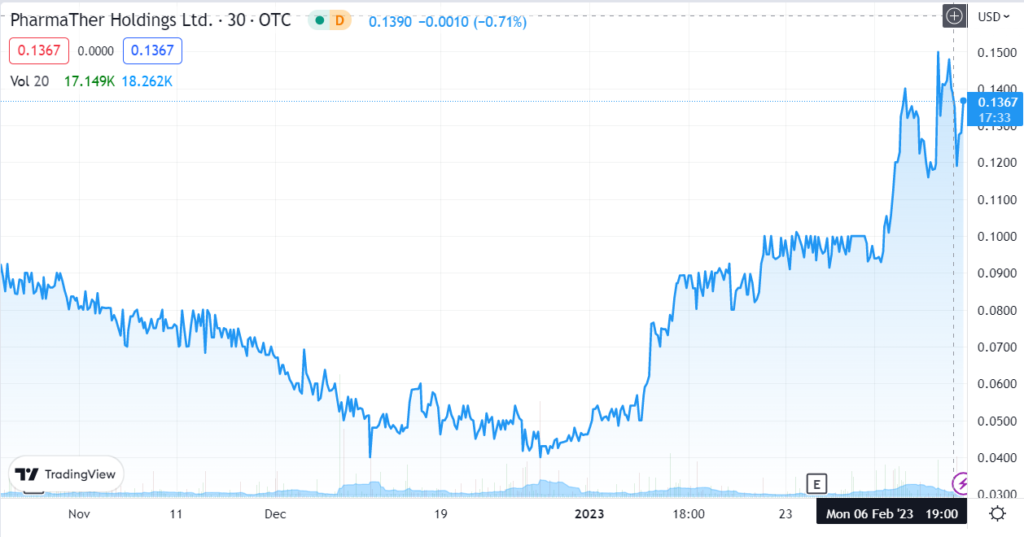
February 3rd. Revive announces it has entered into a research collaboration agreement with PharmaTher Holdings Ltd. (PHRRF) to evaluate the delivery of MDMA using PharmaTher’s novel microneedle patch (“MN-Patch”) delivery technology. Press Release.
CHARTS
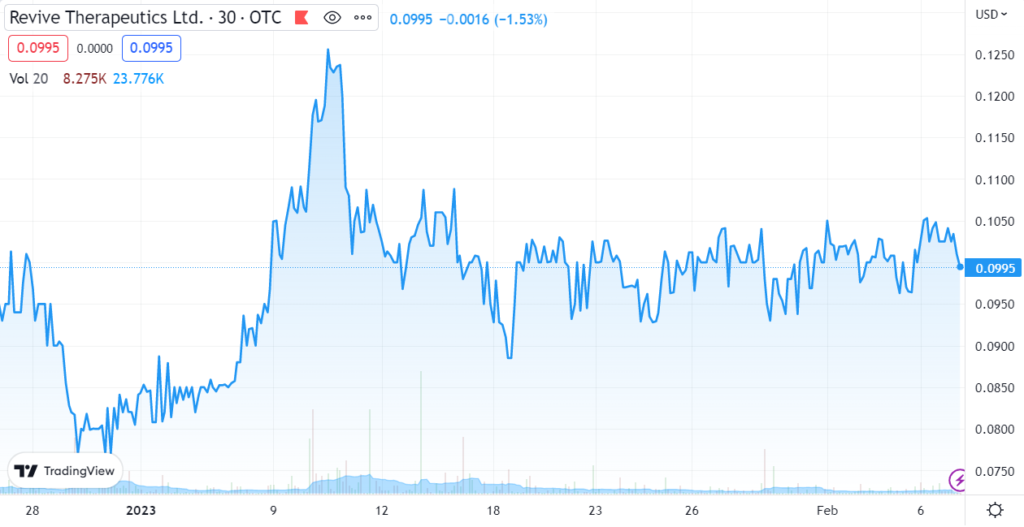
REVIVE (RVVTF)
Neither the Canadian Securities Exchange nor its Regulation Services Provider has reviewed or accepts responsibility for the adequacy or accuracy of this release.
Cautionary Statement
This press release contains ‘forward-looking information’ within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words “could”, “intend”, “expect”, “believe”, “will”, “projected”, “estimated” and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on Revive’s current belief or assumptions as to the outcome and timing of such future events. Forward looking information in this press release includes information with respect to the the Company’s cannabinoids, psychedelics and infectious diseases programs. Forward-looking information is based on reasonable assumptions that have been made by Revive at the date of the information and is subject to known and unknown risks, uncertainties, and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking information. Given these risks, uncertainties and assumptions, you should not unduly rely on these forward-looking statements. The forward-looking information contained in this press release is made as of the date hereof, and Revive is not obligated to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities laws. The foregoing statements expressly qualify any forward-looking information contained herein. Reference is made to the risk factors disclosed under the heading “Risk Factors” in the Company’s annual MD&A for the fiscal year ended June 30, 2022, which has been filed on SEDAR and is available under the Company’s profile at www.sedar.com RVVTF is a news coverage client of Institutional Analyst Inc., see reports for disclaimer and disclosure details.
#RVVTF, $RVVTF, #MDMA, $MDMA, #PHRRF, $PHRRF