Here’s How Provention’s Treatment Works.
Interview With an Actual Provention Bio (PRVB) Clinical Trial Participant.
Conversation Between a Mikayla a Teenager who was at Risk of Developing Type I Diabetes and Jill Rotella from the Nebraska Junior Diabetes Research Foundation.
Jill is a Mother whose 8 year old son developed Type I when he was just 8 years old. So unlike those of us who are primarily interested in Provention succeeding, so we can turn a small pile of dollars into a bigger pile of dollars, her interest is in improving the lives of young children.
This is the way the process works: If a patient tests positive for certain autoantibodies (from a $55 test kit*) that can be detected in a blood sample – odds are they will develop Type I diabetes.
This will eventually lead to a life of daily insulin injections. This daily care, a 24/7 job is difficult for anyone, but particularly hard on teens and/or pre-teens. In her clinical trial, Mikayla underwent a two week Provention treatment process – with the goal of delaying this life of injections, for up to three years. And possibly more.
Mikayla is on year five.
A Conversation with a JDRF Teplizumab Clinical Trial Participant.
Still Formulating an Investment Strategy.
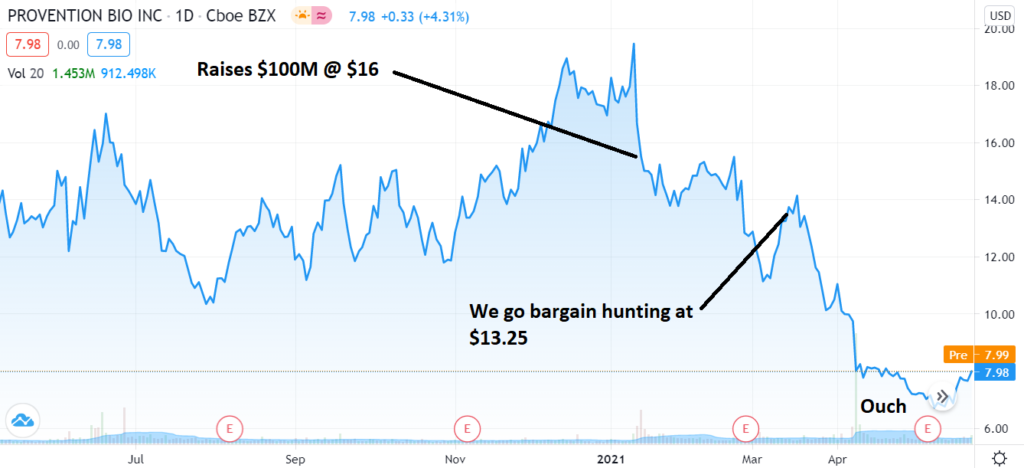
Watching that video makes the chart below even that more disappointing. Our early entry bargain hunting at $13.25 – made us feel smarter than the very smart investors at SVB Leerink and Cantor Fitzgerald & Co. who wrote Provention a check for $100 million at $16 – was, well, too early. Forget that we paid less than them or that is was significantly off its high of $20 in January.
CHART ONE
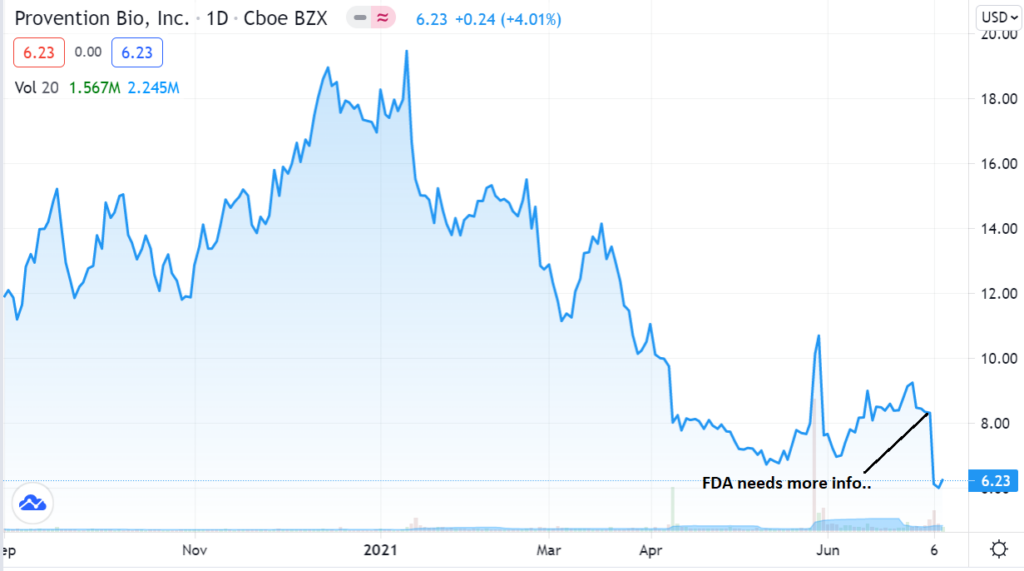
At $6.00 – it doesn’t matter much knowing we had smart money fellow shareholders; Peter Appel with 2.2 million shares, Perceptive Advisors 3.3 million shares, Venrock 3.8 million shares, Blackrock 3.8 million shares and Amgen with 2.5 million shares from their Celiac deal.
CHART TWO
Not being among the cadre of today’s traders, and even with our target looking out a year, we would nonetheless like to enter at the best possible near-term price and at the best possible time. In our opinion the question isn’t whether we should own more Provention – but rather when?
Of course investing in something solely because you wish an unproven treatment will improve lives when approved – while noble – (vs investing in an e-cig company like JUUL) isn’t the smartest thing to do. But when an unproven treatment does get approval and the stock zooms upward, it’s truly a joyous and momentous event. A double barreled win. Which is why we have both fingers crossed and we wait patiently (currently) for an eventual approval.
So we are still strategizing our best wait and see approach. The teplizumab clinical development program has been ongoing over 2 decades, so in a more positive light, the majority of the wait is over. What’s another year, right?
ADVISORY COMMITTEE BRIEFING MATERIALS
* Upwards of 85% of people with T1D have no family history of the disease. That’s why this test is available for everyone. T1Detect
Internet Posting of Information:
Provention Bio, Inc. uses its website, www.proventionbio.com, as a means of disclosing material nonpublic information and for complying with its disclosure obligations under Regulation F.D. Such disclosures will be included on the Company’s website in the “News” section. Accordingly, investors should monitor this portion of the Company’s website, in addition to following its press releases, SEC filings and public conference calls and webcasts.
Forward-Looking Statements:
Certain statements in this press release are forward-looking, including but not limited to, statements relating to the medical need in T1D at-risk patients, the potential therapeutic effects and safety of teplizumab in at-risk T1D patients, the timing and ability of the Company to obtain additional PK/PD data from a PK/PD substudy in the ongoing Phase 3 PROTECT trial and other data and analysis relevant to PK comparability, the potential for these data to address the FDA’s PK comparability considerations, the Company’s belief that the remaining product quality issues cited in the CRL are addressed or can be addressed in the short-term, the FDA review of such data if submitted by the Company, the need for resolution of deficiencies identified at a fill/finish manufacturer used by the Company,and the Company’s plans to address the other matters raised in the CRL including plans to continue working collaboratively with FDA to hopefully secure teplizumab approval . These statements may be identified by the use of forward-looking words such as “likely,” and “may,” among others. These forward-looking statements are based on the Company’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to delays in or failure to obtain FDA approvals for teplizumab or other Company product candidates and the potential for noncompliance with FDA regulations; any inability to successfully work with FDA to find a satisfactory solution to address its concerns in a timely manner or at all, including any inability to provide the FDA with PK/PD data from our ongoing Phase 3 PROTECT study or other data sufficient to support an approval of the BLA for teplizumab; an inability to satisfactorily address other matters cited in the CRL including relating to product quality, fill/finish manufacturer deficiencies identified in a general inspection, safety update required by FDA or any other FDA requirements for an approval of teplizumab the potential impacts of COVID-19 on our business and financial results; changes in law, regulations, or interpretations and enforcement of regulatory guidance; uncertainties of patent protection and litigation; the Company’s dependence upon third parties; substantial competition; the Company’s need for additional financing and the risks listed under “Risk Factors” in the Company’s quarterly report on Form 10-Q for the quarter ended March 31, 2021 and any subsequent filings with the Securities and Exchange Commission. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Provention does not undertake an obligation to update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by applicable law. The information set forth herein speaks only as of the date hereof.