

ILLUSTRATION 3 |MISSION AND STRATEGY
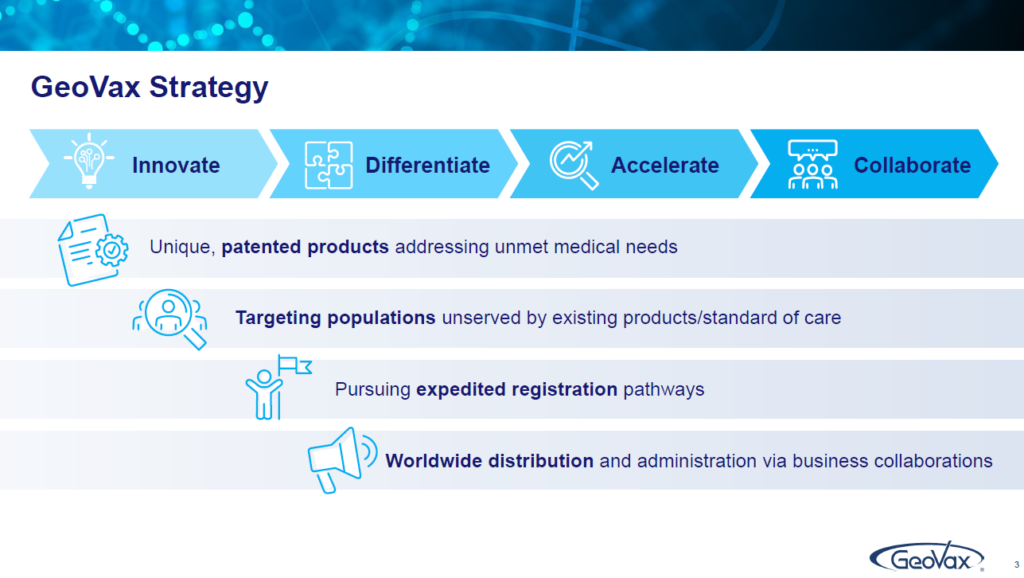
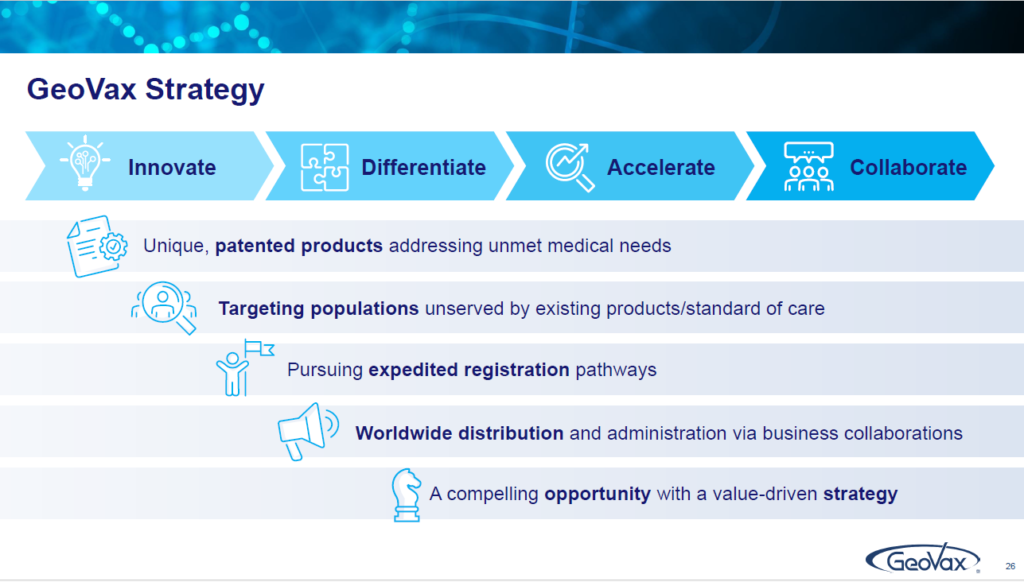
Our mission: One is to innovate. The other one is to differentiate. The other one is to accelerate and finally to collaborate. And that is the basis of the business strategy that we have as a corporation. We want to focus on making sure that we have unique products and technologies that can deliver differentiated products that address the needs of those who are either underserved or not served well by what is existing therapy or either existing vaccine opportunities.
We want to do this in a manner that will accelerate our path to commercialization, revenue development and cash flow, and we recognize that as a small company, even though we’re developing all of our assets for global registration and global opportunity, we cannot distribute and administer those without strong business partnerships and collaborations. That is the basis of everything that we work on and what drives our planning and where our opportunities are.
ILLUSTRATION 4 | MANAGEMENT

I’m not going to dwell on this but we’ve got a lot of experience otherwise we’ve got a lot of old guys and, and we have some younger people also, but you’re seeing some of us here in the room.
ILLUSTRATION 5 | GEOVAX PORTFOLIO
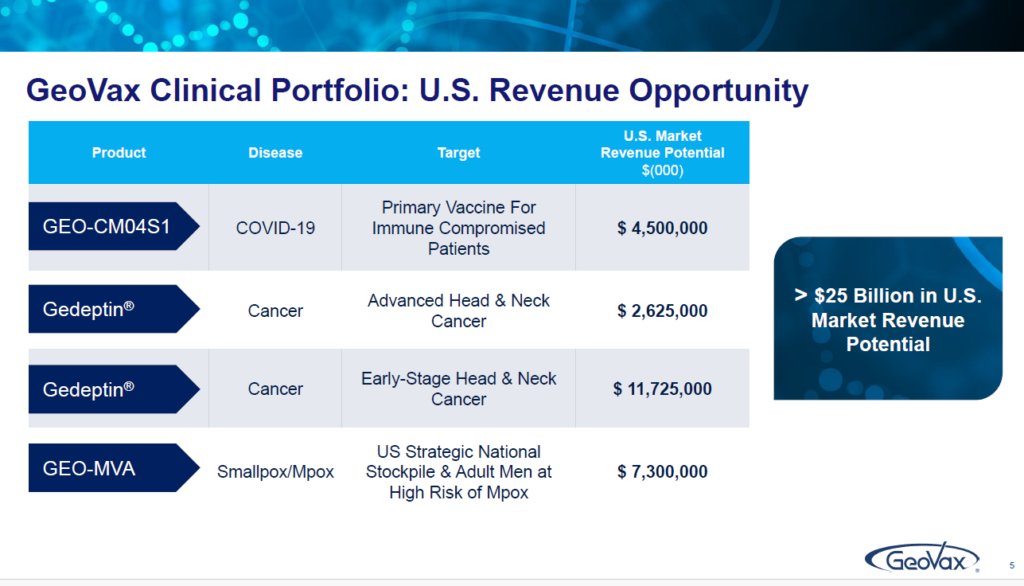
I do want to emphasize that what I’m going to focus on today are areas that are either in the clinic or going into the clinical development. But what I would want to underscore is that in the US alone, these three assets represent over $25 billion in annual revenue opportunity. And so, there’s a lot available for where we are focused on and I’m going to touch on each one of these programs and what they offer up to us and to patients and in future opportunities.

ILLUSTRATION 6-7 | GEDEPTIN FOR CANCER

Starting with oncology, and the medical need for “GDEPT”. You’ll see in a minute what it is and what it does. It’s a brand name of a technology, which is initially being developed for patients with advanced head and neck cancer.
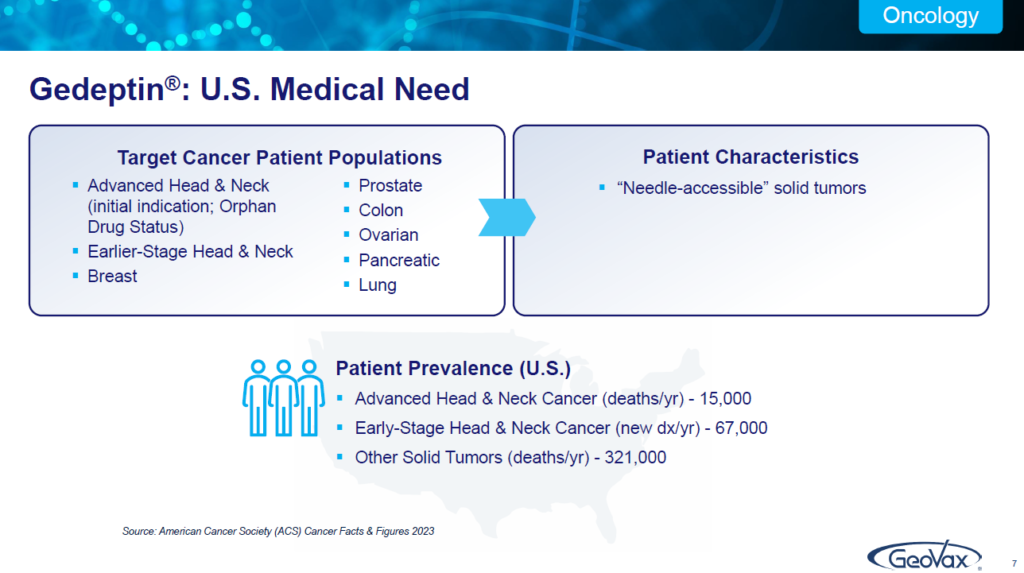
There are 15,000 such patients in the United States every year that unfortunately die, they’ve failed on everything. They’re on palliative care. There’s 67,000 new cases every year of head and neck cancer. We’re focused on that population for whom there has really been nothing for them. We have orphan drug status.
In the current phase two program, which we announced last week we had completed the enrollment of that program, is being funded by the FDA under the FDA orphan drugs clinical trials program.
But our plans are to develop this asset to move quickly. To be able to this is the acceleration to bring it to commercialization by targeting an unmet area of medical need these particular patients and then to work to earlier stage cancer targets both in model therapy as well as in combination therapy in conjunction with immune checkpoint inhibitors and I’ll touch on that, but it’s basically been shown to the technology to be tumor agnostic. So, we’re focused on solid tumors.
ILLUSTRATION 8 | HOW GEDEPTIN WORKS
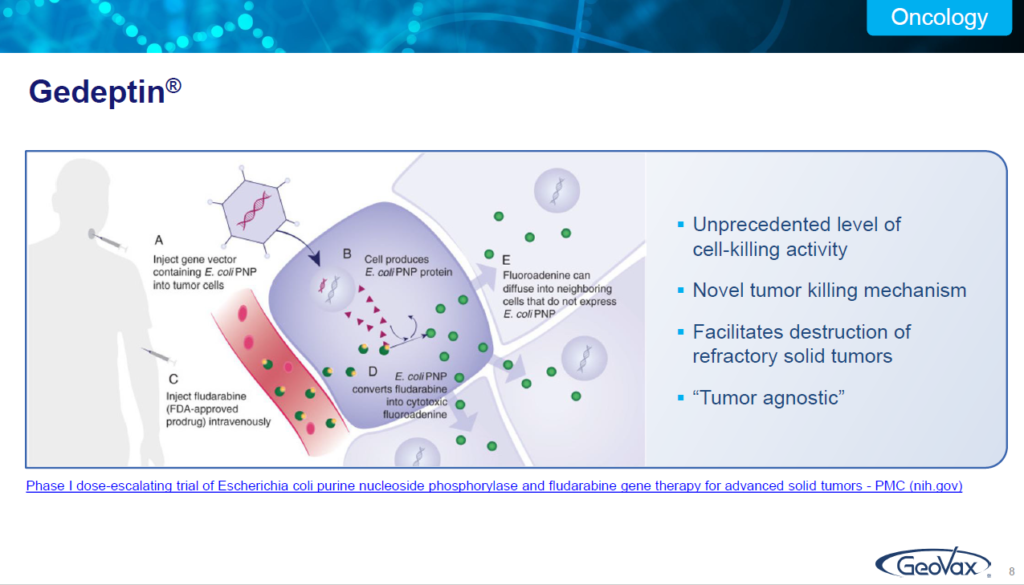
How does it work? I’m not going to get into a lot of discussion here. Just remember A, B, C, D, E. So A is you take a targeted tumor and you inject a particular enzyme, the PNP enzyme into that. B as it converts into the PMP cells. C is you then follow it with administration of a Fludara, fludarabine phosphate.
Fludara or fludarabine phosphate is an approved hematologic chemotherapeutic agent, which in and of itself does nothing with solid tumors.
But if it encounters a tumor that has been treated with the PNP enzyme, it converts into a highly cytotoxic compound called fluoroadenine that is destructive to the tumors. If there is no presence of the PNP enzyme in the tumor it simply passes through. It’s otherwise, very safe.
So that is the basis of the technology that we are utilizing is to go after solid tumors in a variety of different manners and at different stages, as well as in combination therapy with ICIs.
ILLUSTRATION 9 | GEDEPTIN TARGET MARKET
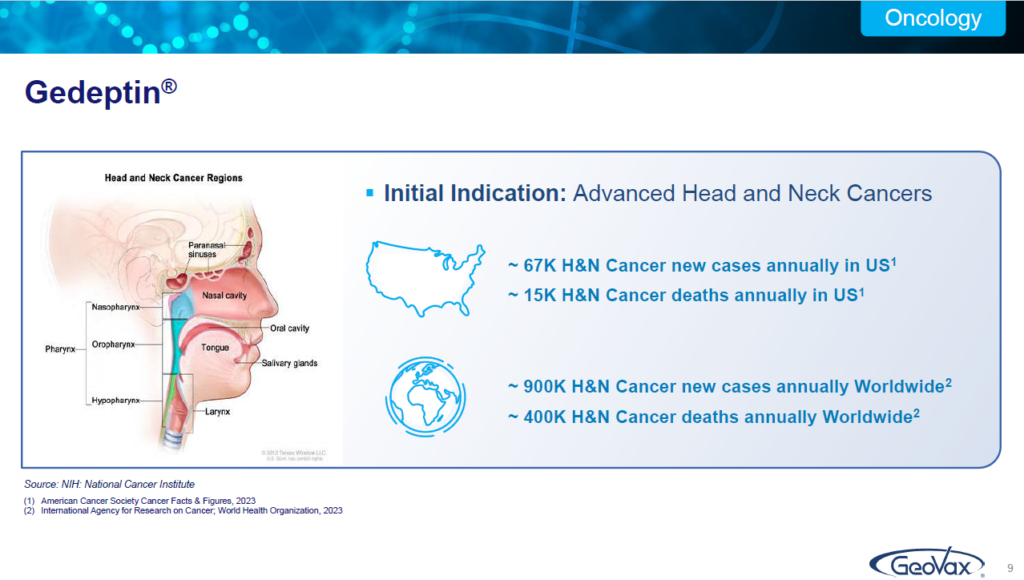
I mentioned some of these statistics in the profile slide. Worldwide there are about 400,000 individuals who die annually of head neck cancer, this is from the WHO. This is our initial indication, going after this particular population but, with a focus on bringing it to earlier stage therapy and opportunities as we go forward.
ILLUSTRATION 10 | CLINICAL TRIAL DATA
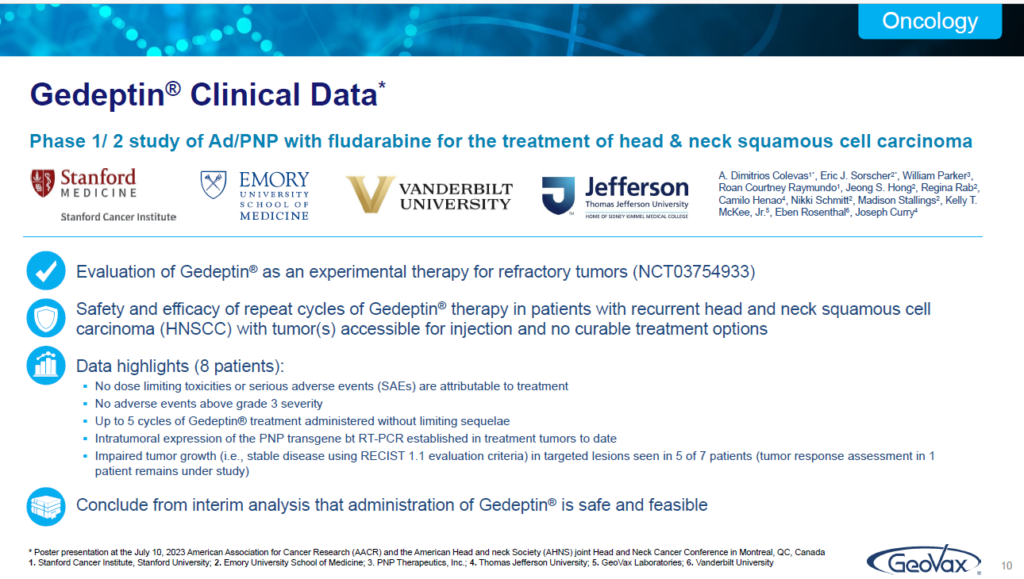
In the past Summer, we presented at the annual international meeting in Montreal of the AACR (American Association Cancer Research and Association of Head and Neck Society). We presented data on the current trial that showed that it is in fact extremely safe. That’s always very important.
But perhaps most important, is that Gedeptin does provide consistent reduction in the tumors and that’s what we’re focused on doing in our current clinical program. Our goal and in guidance from the FDA for the current trial has not been survival. We’re not trying to rescue these patients. These patients, unfortunately, are on palliative care. They’re not going to survive.
But if we can provide them a reduction in the tumors so that they can perhaps swallow food better or if they could perhaps speak better. We’re focused on providing them a much better quality of life for their stage of life. The objective measures are the reduction on the tumor size through the treatment.
But in reality, we recognize that this is not a rescue strategy. At this stage. We’re really focused on a very critically important group which are individuals who otherwise have nothing and it’s a horrible disease if you’ve ever interacted with people who’ve had head and neck cancer.
ILLUSTRATION 11 | CLINICAL TRIAL PROGRESS
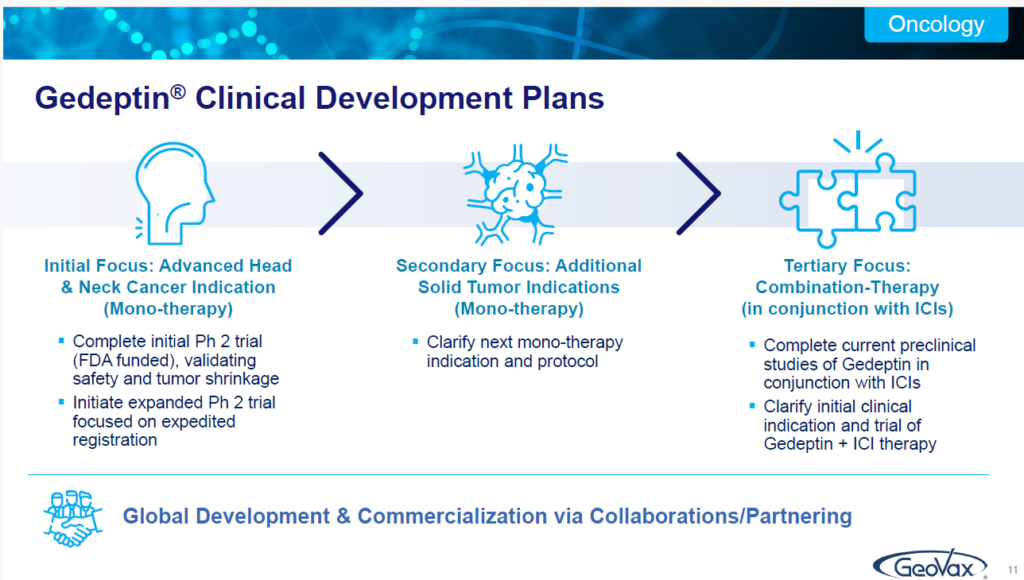
So, following that, we will then focus on an expanded phase two. That’s what our team is working on now, with the focus on interacting with the FDA to discuss the basis or the opportunity for an expedited process based upon an expanded phase two type trial.
And then we’ll also develop additional mono therapy use. But, at the same time, we’ve been supporting animal trials and we expect to be going forward in the next year, or a little bit more than a year, with an IND for combination therapy in conjunction with immune checkpoint inhibitors.
In that regard, we’ve seen some very encouraging data that demonstrates that if you utilize a checkpoint inhibitor in conjunction with Gedeptin, you end up seeing much greater, more consistent, successful performance of the checkpoint inhibitor. We think there’s a tremendous amount of opportunity with this technology.
ILLUSTRATION 12-13 | COVID VACCINE FOR IMMUNOCOMPRIMISED

We have a next generation COVID 19 vaccine. Now everybody says I don’t want to hear any more about COVID-19. We spent the last several years dealing with that.
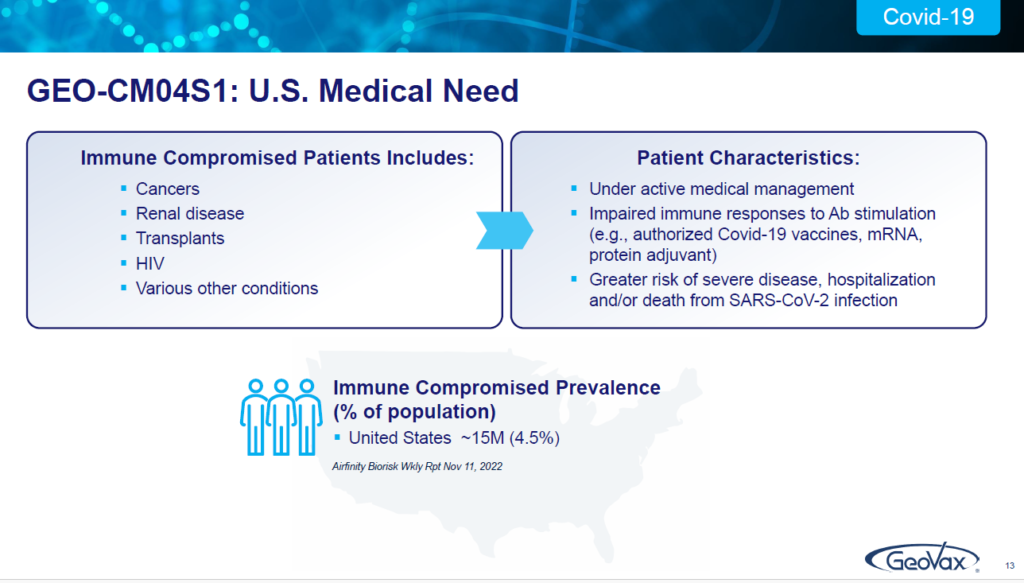
You may not be aware of it, but there are 15 million people in the United States; there are 240 plus million people worldwide, who, because they have certain medical conditions…they may have blood cancers, they may have renal disease, they may be HIV positive. They may have sickle cell anemia, any number of of conditions that have depleted their body’s ability to respond to antibody stimulation.
These are individuals who are not being adequately served by the current authorized vaccines for COVID 19 or the monoclonal antibodies. And that’s a real challenge for these patients. There’s been nothing out there to deal with them. Some have a very high risk of severe disease, hospitalization and death.
And that’s what we’re targeting. And we’re doing that for a couple of reasons. One, obviously being a small company. We cannot go toe-to-toe with very large players; but, if we can utilize our technology and go after a portion of the population, for whom the current vaccines are inadequate, and don’t work, then we can own that space and have a very nice business. That’s our business plan and what we’re focused on.
ILLUSTRATION 14 | IMPORTANCE OF T-CELLS FOR IMMUNITY
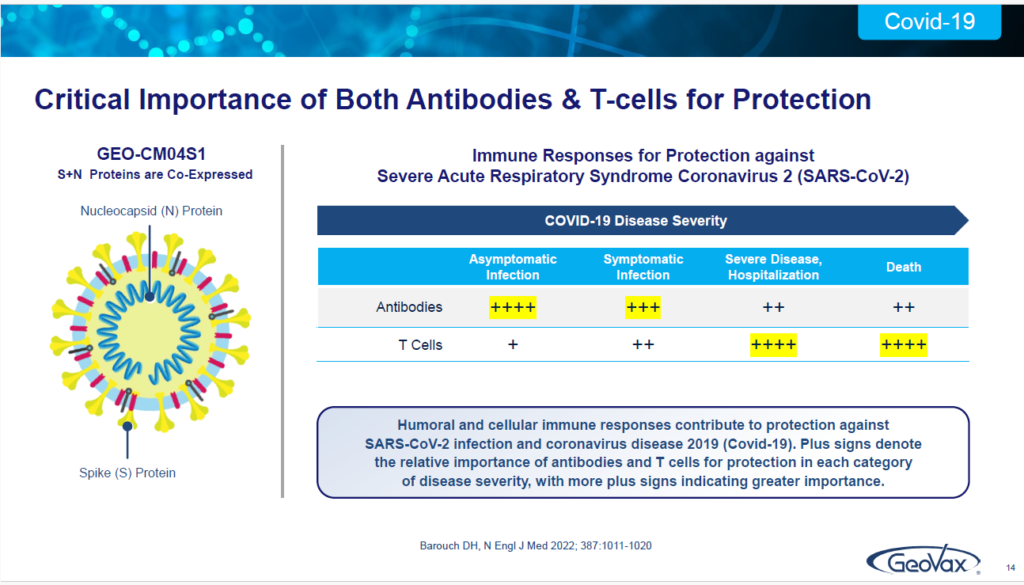
Now we do this because we take the SARS-CoV-2 virus and, instead of just utilizing the Spike protein, which is what all the other vaccines are doing to induce a strong antibody response… remember that I mentioned these patients — their bodies do not respond to antibody stimulation.
What we also do is we include another component of the virus called the Neucleocapsid or N-protein, which is recognized for being conserved, meaning it isn’t changed or affected by the virus — as you see with a spike protein, as new variants come along and evolve.
So, it’s conserved across all coronaviruses. So, we have a dual antigen approach. This is the only product in clinical development that has a dual antigen approach. This our product and in this slide, on the right side, this is out of a publication from 2022, in the New England Journal of Medicine by Dan Barouch, out of Harvard.
He highlighted that antibodies are very important in the early stage of infection. But, if you really want to reduce severe infection, hospitalization and the risk of death, you have to induce and utilize T cells. And, in our Covid-19 vaccine, that’s what we get and what we induce, by taking this dual antigen approach.
We induce the antibodies, but with the nucleocapsid protein included, we also induce a very strong T cell response. These data have been published in September of last year, in Vaccines, demonstrating that our vaccine, without having to reconfigure it as you have seen with all the other vaccines that are out there, the mRNA etc.
We’ve demonstrated protective immunity from the original ancestral Wuhan strain all the way through Delta and Omicron, the XBB.1.5. People keep asking me have you done JN.1 yet? The answer is “not yet, but stay tuned”…because we continue to demonstrate that our construct does not have to be reconfigured.
It’s demonstrating, thus far, twice the durability that we’re seeing with mRNA with a very strong a protective immunity response.
ILLUSTRATION 15 | VACCINE WHICH IS ROBUST, DURABLE AND VARIANT AGNOSTIC
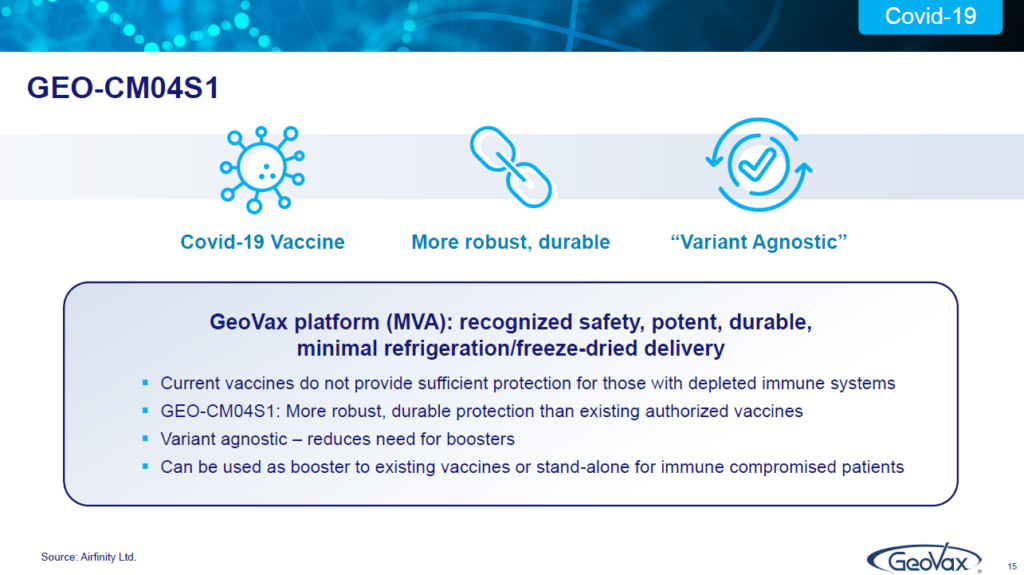
What are our plans here? is that “one”, we think that by doing this, it’s been basically shown to be variant agnostic. We haven’t had to reconfigure it, which means you can reduce the need for all the boosters that people are having to have. And, it could also be a booster on top of, for instance, mRNA vaccines which we think would give a more robust, more durable poster.
ILLUSTRATION 16 | VACCINE TARGET MARKET
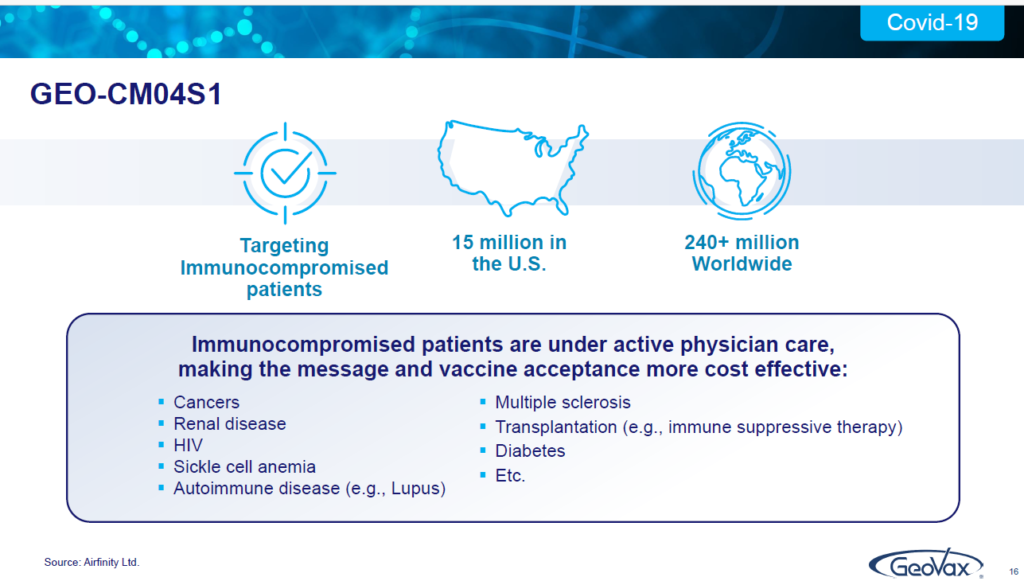
ILLUSTRATION 17 | VACCINE CLINICAL TRIAL PROGRESS

Let me just go here. In fact, we have three phase two trials that are underway right now, one is immunocompromised patients receiving stem cell transplantation. This is a very high risk recognized group that now currently in four sites, in the United States: at Fred Hutchinson Center, University of Massachusetts Wake Forest and Eastern Carolina Medical Center, all currently actively enrolling. We have immunocompromised patients who have chronic lymphocytic leukemia, again a very high-risk group from this blood disease.
That study is doing a direct randomized comparison of either our vaccine or the Pfizer vaccine as a booster for patients who have CLL or chronic lymphocytic leukemia. That one is moving forward. We expect in 2024 to reach the point of an interim report on the date on there. So, we’re looking forward to that.
Finally, as a booster among healthy individuals who received initially the mRNA vaccine, we have a fully-enrolled, as of October of this past year. It’s 63 patients.
ILLUSTRATION 18 | VACCINE DEVELOPMENT PLAN

So what will occur during 2024 is, we’ll start seeing the data and reporting the data out of that patient who are now coming in for different blood draws as you can imagine. I’ll also just underscore, for those of you who may sometimes wonder, “how do you afford all this?”, the immunocompromised CLL trial is actually being funded by a private family foundation.
So, we’re benefiting from their support and investment behind that. The data is ours to use but, that’s a nice way to have a program going.
So, we’ve got Gedeptin, which has been recently completed in the enrollment which was funded by the FDA, and we have the CLL trial being funded by a private family foundation. It’s nice to have that.
Our development plan is to basically validate the differentiation? Remember how important that is — I pointed out, we expect to be able to demonstrate a broader, more durable type of response and to be the preferred vaccine for those individuals who fall into immunocompromised groups.
By doing that, we anticipate the opportunity for an expedited registration process. So, again, it goes back to those four terms I’ve mentioned and we’re very active in discussions already in about commercialization partnerships now on a global basis. Because, again, we have worldwide rights for this technology.
ILLUSTRATION 19-20 | MVA VACCINES FOR MONKEYPOX & SMALLPOX


There’s one vaccine in the world that is authorized for monkey pox, as well as for smallpox, and that’s called Modified Vaccinia Ankara, “MVA”.
What’s interesting is that MVA is the basis of, or the platform, what we utilize as our vaccine platform for all of our infectious disease vaccines. That includes the next generation COVID-19 vaccine. In fact, it’s the only vaccine platform that in and of itself is a vaccine. mRNA is not. It’s a platform, but it’s not a vaccine. It does nothing until you insert particular antigens in it. Adenovirus, which people at J&J and AstraZeneca might use, is not. It’ll do nothing until you decide what antigens to insert.
But, MVA in and of itself prevents monkey pox and smallpox. We’ve demonstrated, in a publication in August of 2022, that our candidate which is in clinical testing, for COVID 19, which we refer to as CM04S1, also prevents provides protective immunity against monkey pox!
In some parts of the world, that becomes very important. But, what we also recognize is that there’s only a single supplier worldwide of MVA as a standalone vaccine, a small Danish company. They don’t have the capacity or the capability to manufacture in a manner to meet the world demand.
ILLUSTRATION 21 | THE UNMET NEED FOR MVA VACCINES FOR MONKEYPOX & SMALLPOX
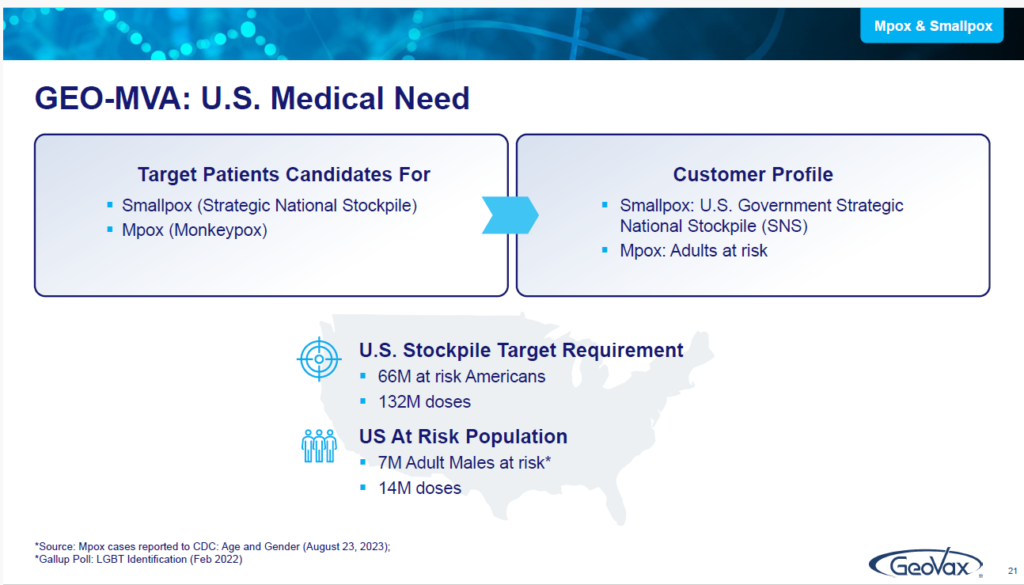
So, we looked at that and, in late 2022, we acquired the rights from the National Institutes of Health (NIH) to be able to use MVA to develop it as a standalone vaccine for both monkey pox and smallpox.
And, we intend to do that in a manner using a new advanced manufacturing system that will enable us to respond quickly with greater vaccine being produced with a higher yield, able to address epidemics and pandemics. The purpose of this is that this could very likely, be our first pathway to reaching revenue production and cash flow, by moving forward with an MVA standalone vaccine. It also is a priority for the USA.
The objective, relative to GEO-MVA, for this year, is to report what we end up clarifying with regulatory authorities as to the required regulatory pathway. There are a lot of patients and we want to eliminate the need for stockpiling. Throughout the world, in 2022 and, even just today, we received a communication on the Democratic Republic of the Congo about a big outbreak that they’re having there.
They’re in great need of MVA vaccine, but there’s not enough product to go around. People are aware of what we’re developing here and what our intentions are. We expect to be the first US-based supplier of MVA as a standalone vaccine against both monkey pox and smallpox.
Keep in mind, Smallpox is important because it’s number one on the list of bioterrorism. That’s how the Strategic National Stockpile got started — because of the concerns about certain biological threats. And following smallpox is hemorrhagic fever viruses, having very high fatality rates.
If you look into GeoVax, you’ll see that we’ve also been demonstrating, not only additional new patents being issued regarding of how we approach the hemorrhagic fever vaccines, but also recent publications of the data of our respective vaccine product candidates.
ILLUSTRATION 22-23 | MANUFACTURING MVA VACCINES FOR MONKEYPOX & SMALLPOX


Finally, I want to underscore — I mentioned that the current manufacturer of MVA that is used throughout the world today, uses an antiquated, old manufacturing system. We initiated, a few years ago, a process to try and transform to a new advanced manufacturing system, that would be able to be produced products in real time to respond to epidemics and pandemics.
We ended up making several announcements, last year, one was to demonstrate the data that showed that we could produce using a continuous cell line, an avian cell line which means it comes from chickens or ducks, etc. That we could produce in that regard.
We ended up disclosing what that cell line was when we completed the license of that and, we announced that in September. We also established a relationship with a contract manufacturer for producing MVA based products a CDMO that is well recognized and, performs a lot of work for the US government with MVA manufacturing.
So, we’ve got all the pieces in place. And this represents a major objective for this year. This investment isn’t necessarily that exciting to the investment community, because investors typically prefer to think about products.
But remember everything we do that’s infectious disease related is based upon MVA, which is why transitioning to a continuous avian cell line process is nothing less that transformative related to vaccine manufacturing and supply.
Moving to an advanced manufacturing process means that we will then be able to leapfrog over anyone else that utilizing MVA and be out there, as not only being able to produce products that look good, let’s say in the lab, but…when you demonstrate and validate them clinically, you can then produce in rapid time what is needed worldwide.
ILLUSTRATION 24 | MILESTONES, CATALYST & SUMMARY

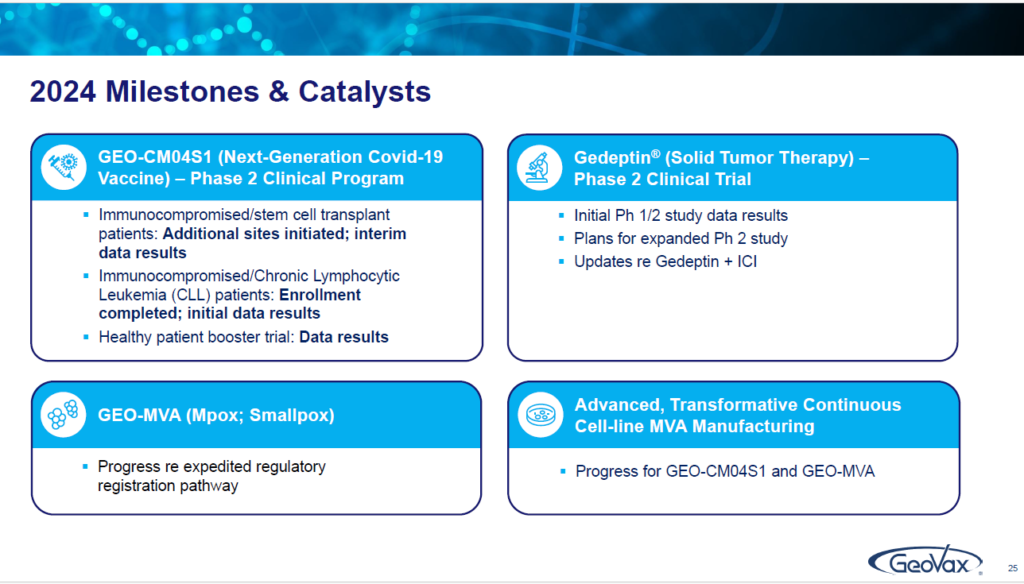
So, if we then simply look at what we’re focused on this year are the four things that I’ve touched on:
Our next generation COVID-19 vaccine in the trials. The data that will be coming out Gedeptin. Reporting on what we see coming out of the trial that we’ve just completed the enrollment, as well as, our plans for taking it forward, including in conjunction with immune checkpoint inhibitors. Next is MVA or what we call GEO (for GeoVax)-MVA, as our vaccine against monkey pox or smallpox, and, our progress in the advanced MVA manufacturing system. That’s what we’re all about.
Just remember, it all goes back to the concept of innovate, differentiate, accelerate and collaborate…because in the end, for us to generate the value for our shareholders and to have the fun we want to have, by having the jobs we enjoy, we’ve got to be able to make sure that these products are not only distributed but, that those products are able to be administered on a global basis. With that, I’m finished with the presentation.
SUMMARY
Forward-Looking Statements
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.
Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
Bolding for emphasis by Editors of the Biotech Stock Review, not the company.












