– Addition of leading scholar and KOL in acute respiratory distress syndrome (ARDS) to ARDS Scientific Advisory Board supports continued progress of Citius’ novel induced mesenchymal stem cell (i-MSC) program –
Related: CITIUS NEWS ARCHIVE
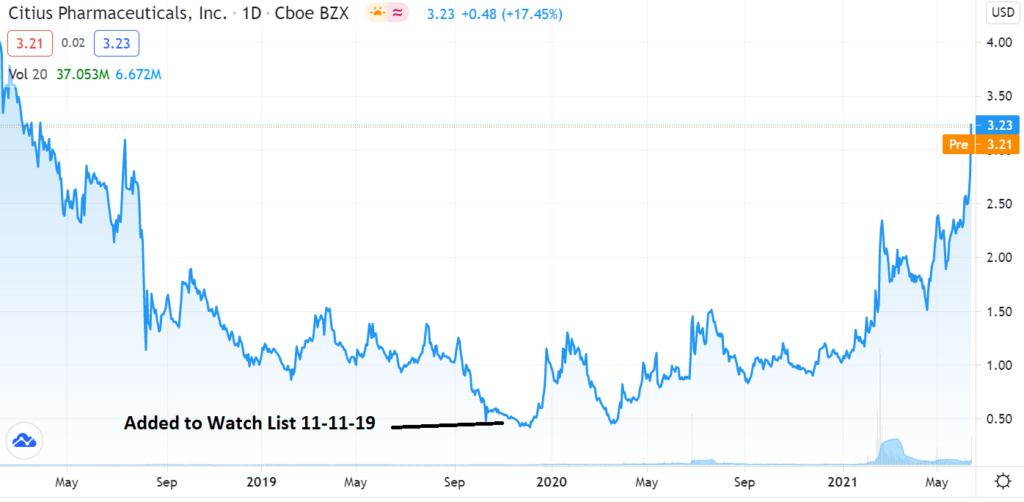
Related: Adding Citius (CTXR) $0.55 to Watch List.
Related: Adding Citius Pharma (CTXR) $1.29 to the 2021 Biotech Portfolio.
Related: Citius (CTXR): Halt For Superiority On The Horizon? (SeekingAlpha).
(ARDS is an inflammatory process leading to build-up of fluid in the lungs and respiratory failure. It can occur due to infection, trauma and inhalation of noxious substances. ARDS accounts for approximately 10% of all ICU admissions and almost 25% of patients requiring mechanical ventilation.)
CRANFORD, N.J. , June 15, 2021 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) (Nasdaq: CTXR), a biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products with a focus on anti-infective products in adjunct cancer care, unique prescription products and stem cell therapy, today announced that Dr. John Laffey , a leading scholar in Acute Respiratory Distress Syndrome (ARDS) and the use of cutting-edge therapies to treat acute lung disorders, has been appointed to its ARDS Scientific Advisory Board (SAB), effective immediately.
Dr. Laffey joins noted colleagues, Dr. Michael A. Matthay , Dr. Lorraine B. Ware , Dr. Mitchell M. Levy , and Dr. Perenlei Enkhbaatar on the ARDS SAB. Each member of the board is a recognized key opinion leader (KOL) with extensive clinical trial expertise in the treatment of acute lung disorders.
“We are delighted to welcome John to our distinguished ARDS Scientific Advisory Board and look forward to leveraging his deep insights and decades of hands-on experience with ARDS to advance our proprietary i -MSC program,” stated Myron Holubiak , President and Chief Executive Officer of Citius.

“Dr. Laffey is a highly accomplished and recognized expert in acute lung disorders. His cutting-edge research into the use of gene and stem cell-based therapies to treat ARDS will provide Citius with an important perspective as we explore the potential of this exciting new therapeutic area. Development of our i -MSC master cell bank for future trials is underway, and I am confident that John’s expertise and guidance will complement our SAB and help direct our clinical efforts,” concluded Mr. Holubiak.
Dr. John Laffey is Professor of Anesthesia and Intensive Care Medicine at the School of Medicine of the National University of Ireland (NUI Galway), and a Consultant in Anesthesia and Intensive Care Medicine at Galway University Hospitals. He is Director of Clinical Research at Saolta Hospital Group and NUI Galway. Dr. Laffey’s primary focus is on the investigation of therapeutic strategies for ARDS. He founded and leads the lung biology group at NUI Galway. His research groups are currently conducting studies into the pathogenesis of ventilation induced lung injury and sepsis induced Acute Lung Injury. The current focus of his research is on the investigation of the therapeutic potential of gene and stem cell-based therapies for acute lung injury.
Dr. Laffey is also a principal investigator at the National Centre for Biomedical Engineering Sciences, and a Clinical Trials Investigator at the HRB Clinical Research Facility at NUI Galway. He has acted as principal investigator or co-principal investigator in multiple clinical trials in the fields of anesthesia and critical illness.
Sign Up For Citius Updates Here.
Additional members of the ARDS Scientific Advisory Board
Michael A. Matthay , MD , Professor of Medicine and Anesthesia at the University of California at San Francisco (UCSF), a Senior Associate at the Cardiovascular Research Institute, and Associate Director of the Critical Care Medicine at UCSF. Dr. Matthay’s basic research has focused on the pathogenesis and resolution of ARDS, with an emphasis on translational work and patient-based research, including clinical trials. Dr. Matthay’s recent research has focused on the biology and potential clinical use of allogeneic bone marrow-derived mesenchymal stromal cells (MSCs) for ARDS. He is currently leading the “Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (STAT),” a United States Department of Defense supported study of MSCs for ARDS.
Lorraine B. Ware , MD , Professor of Medicine and Ralph and Lulu Owen Endowed Chair, Professor of Pathology, Microbiology and Immunology, Vanderbilt University ; Director, Vanderbilt Medical Scholars Program. Dr. Ware’s comprehensive bench-to-bedside research program centers on the pathogenesis and treatment of sepsis and acute lung injury with a current focus on mechanisms of lung epithelial and endothelial oxidative injury by cell-free hemoglobin. Dr. Ware is also a lead investigator for the “Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (STAT)” study.
Mitchell M. Levy , MD , Chief, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, The Warren Alpert Medical School of Brown University , where he is Professor of Medicine. Dr. Levy also serves as Medical Director of the Medical ICU at Rhode Island Hospital. He has been an investigator on numerous pharmacologic and biologic trials intended to treat sepsis, cardiovascular and pulmonary pathology. He has expertise in trial design, clinical trial execution and trial management and is one of the three founding members of the Surviving Sepsis Campaign (SSC). Dr. Levy is Past-President of the Society of Critical Care Medicine (2009).
Perenlei Enkhbaatar, MD, PhD, FAHA, Charles Robert Allen Professor of Anesthesiology at The University of Texas Medical Branch where he is also Director of the Translational Intensive Care Unit. He is a leading authority on the pathophysiology of acute lung injury and has focused his research on effective therapeutic approaches with special emphasis on tissue regeneration and stem cell biology. Dr. Enkhbaatar has played a leading role in the growing body of knowledge related to basic mechanisms of disease in acute lung injury and acute respiratory distress syndrome. His research advances have been presented at international conferences and in the publication of more than three hundred abstracts, one hundred and fifty-four original manuscripts in leading peer-reviewed journals, and twelve book chapters. In recognition of his contributions to the field of acute lung injury, Dr. Enkhbaatar has been selected to serve on the Editorial Boards of leading journals in the fields of trauma and acute lung injury.
About Acute Respiratory Distress Syndrome (ARDS)
ARDS is an inflammatory process leading to build-up of fluid in the lungs and respiratory failure. It can occur due to infection, trauma and inhalation of noxious substances. ARDS accounts for approximately 10% of all ICU admissions and almost 25% of patients requiring mechanical ventilation. ARDS is a frequent complication of patients with COVID-19. It is sometimes diagnosed as pneumonia or pulmonary edema (fluid in the lungs from heart disease). Symptoms of ARDS include shortness of breath, rapid breathing and heart rate, chest pain (particularly while inhaling), and bluish skin coloration. Survivors of ARDS are often left with severe long-term illness and disability and a decreased quality of life is common.
About Citius Pharmaceuticals, Inc.
Citius is a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products, with a focus on anti-infectives in adjunct cancer care, unique prescription products, and stem cell therapy. The Company’s lead product candidate, Mino-Lok ® , an antibiotic lock solution for the treatment of patients with catheter-related bloodstream infections (CRBSIs), is currently enrolling patients in a Phase 3 pivotal superiority trial. Mino-Lok® was granted Fast Track designation by the U.S. Food and Drug Administration (FDA). Through its subsidiary, NoveCite, Inc., Citius is developing a novel proprietary mesenchymal stem cell treatment derived from induced pluripotent stem cells (iPSCs) for acute respiratory conditions, with a near-term focus on Acute Respiratory Distress Syndrome (ARDS) associated with COVID-19. For more information, please visit www.citiuspharma.com .
Safe Harbor
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “plan,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: risks and uncertainties relating to the results of research and development activities, preclinical and clinical testing, and the early stage of products under development, including our induced mesenchymal stem cell program for the treatment of ARDS; our ability to successfully undertake and complete clinical trials and the results from those trials for our product candidates; the estimated markets for our product candidates and the acceptance thereof by any market; the ability of our product candidates to impact the quality of life of our target patient populations; our need for substantial additional funds; market and other conditions; risks related to our growth strategy; patent and intellectual property matters; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our dependence on third-party suppliers; our ability to procure cGMP commercial-scale supply; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further impacted by Covid-19. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”) filings which are available on the SEC’s website at www.sec.gov , including in our Annual Report on Form 10-K for the year ended September 30, 2020 , filed with the SEC on December 16, 2020 and updated by our subsequent filings with the SEC. These forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law. Citius is a client, please see numerous reports for disclosure and disclaimer details.
Investor Relations for Citius Pharmaceuticals:
Andrew Scott
Vice President, Special Projects
T: 908-967-6677 x105
E: ascott@citiuspharma.com
Ilanit Allen
Vice President, Corporate Communications and Investor Relations
T: 908-967-6677 x113
E: iallen@citiuspharma.com