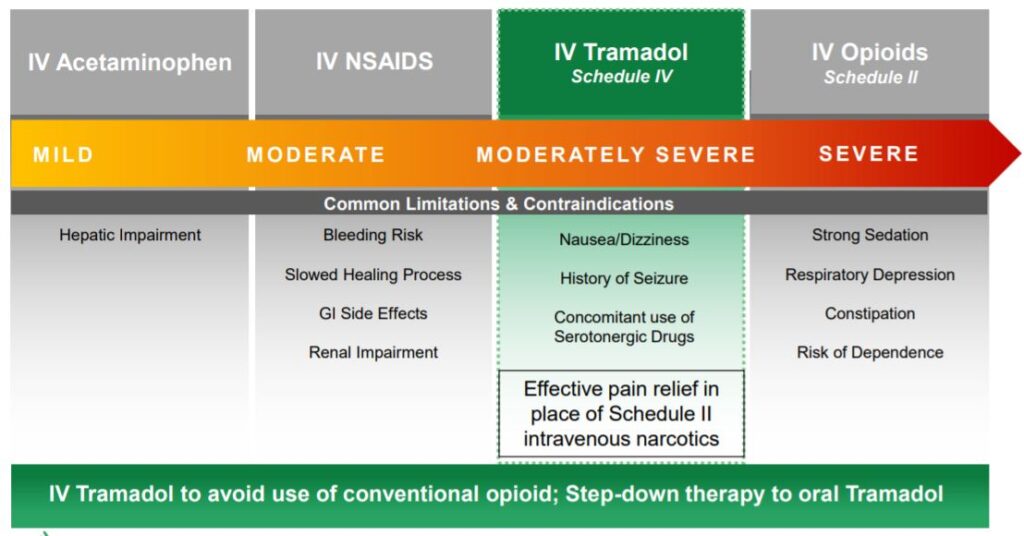
IV tramadol for the management of moderate to moderately severe pain in adults in a medically supervised health care setting.
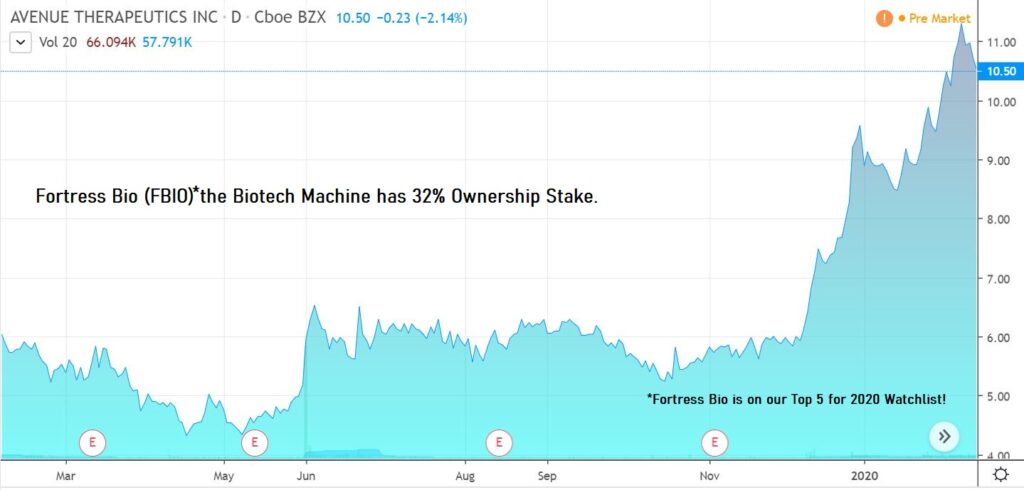
$2.00 to $10 in 16 months isn’t bad! Of course, as with all speculative ideas, one can invest with minimal hard information ($2.00) or invest when a number of questions have been answered ($10) or invest when all questions ($??) have been answered. 🙂
AVENUE THERAPEUTICS POWERPOINT

NEW YORK, Feb. 13, 2020 (GLOBE NEWSWIRE) — Avenue Therapeutics, Inc. (NASDAQ: ATXI) (“Avenue”), a company focused on the development of intravenous (“IV”) tramadol for the U.S. market, today announced that the U.S. Food and Drug Administration (“FDA”) has accepted for review Avenue’s New Drug Application (“NDA”) for IV tramadol for the management of moderate to moderately severe pain in adults in a medically supervised health care setting. The FDA set a Prescription Drug User Fee Act (“PDUFA”) goal action date of October 10, 2020.
“The NDA submission acceptance is another important step toward bringing IV tramadol to patients and their healthcare providers in the U.S.,” said Lucy Lu, M.D., Avenue’s President and Chief Executive Officer. “We look forward to working with the FDA as they evaluate our application.”
RELATED: Biotech 5 Pack. 5 Biotech Stocks We Expect to Double in 2020.
About Avenue Therapeutics
Avenue Therapeutics is a specialty pharmaceutical company whose mission is to develop IV tramadol, a potential alternative that could reduce the use of conventional opioids, for patients suffering from acute pain in the U.S. Avenue is headquartered in New York City and was founded by Fortress Biotech, Inc. (NASDAQ: FBIO). For more information, visit www.avenuetx.com.
Forward-Looking Statements
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks related to us obtaining regulatory approval from the FDA for our product candidate, risks relating to our growth strategy; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
Contacts:
Jaclyn Jaffe and William Begien
Avenue Therapeutics, Inc.
(781) 652-4500
ir@avenuetx.com
FBIO, $FBIO, ATXI. $ATXI












