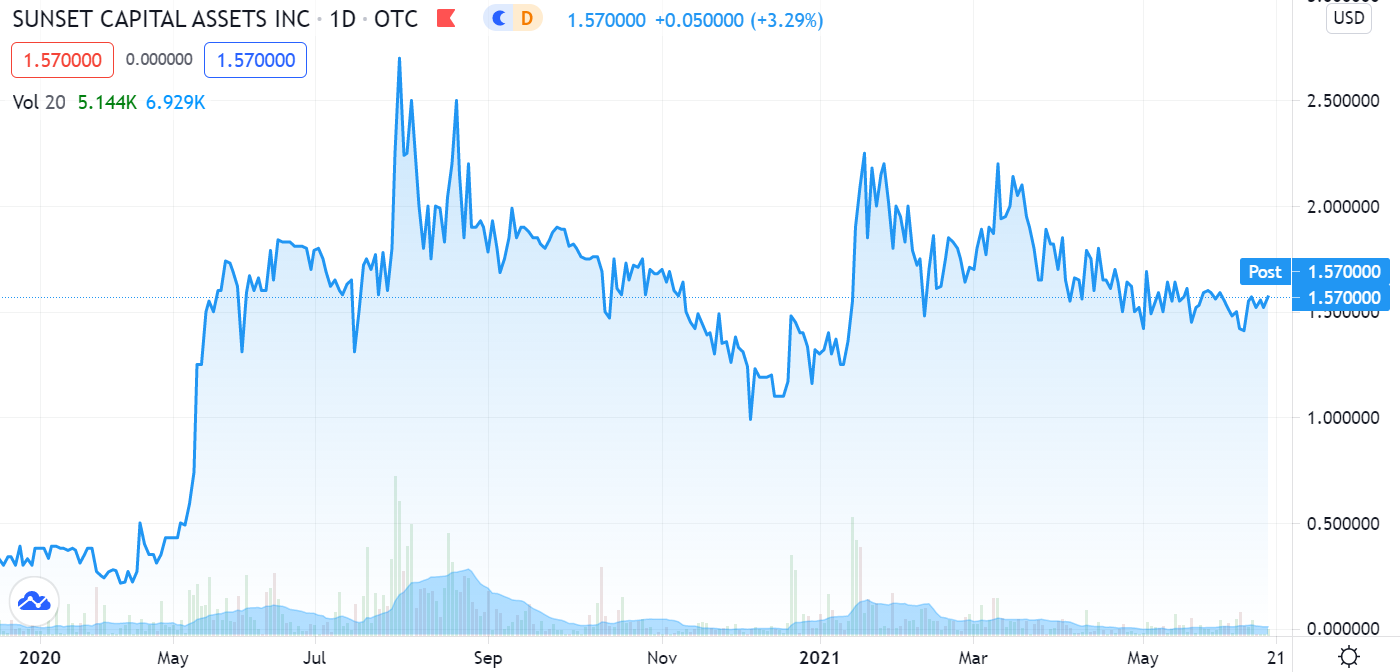
Relatively quiet for most of the year. Aphex BioCleanse, our favorite Super Bug sanitizer company, made two back-to-back announcements – signaling things are about to heat up in their quest to dominate a billion dollar industry.
Aphex BioCleanse Systems Acquires BioFoam(TM) Business and Patented Cleaning Technology; Appoints BioFoam(TM) Founder and Inventor, Scott Smith, as Chief Sustainability Officer
The addition of Smith, BioFoam™ customers, and patented Open-Cell Foam cleaning technology to its existing, high-performance cleaning products, solidifies Aphex as a technology leader in its field.
BioFoam™ was originally developed in 2002 for the U.S. military as a lotion-infused high surface area applicator for neutralizing chemicals on human skin. In 2010, this technology was used by BP America in the cleanup of the BP oil spill in the Gulf of Mexico. Additionally, BioFoam™ was validated by the Center for Disease Control’s (CDC) Environmental Legionella Isolation Techniques Evaluation (ELITE) Labs in 2018 for water sampling for pathogens.
RELATED: Adding Aphex BioCleanse Systems (SNST) to CoronaVirus Watch List.
The product line Aphex is acquiring includes BioFoam™ face shields (BioShield™), Wipes (BioWipe™), Mitts (BioMitt™), and Sponges (BioSponge™), and a Hand Sanitizer and Surface Cleaner (BioWash™). These offerings are reusable, biodegradable, and water-immersible with anti-microbial properties.
“These unique products fit perfectly with our portfolio of offerings as they align with our mission to preserve and protect human health and the environment,” said Aphex President and CEO David J. Weaver. “The old way of sanitizing is ineffective and leaves live germs on surfaces. Our company is here to bring sanitization technology into the modern era with the best available technology. Our goal is to add shareholder value with all of our partnerships and acquisitions and there is nothing more valuable that we could have added than this game-changing technology. We look forward to showcasing the true value proposition of these newly acquired assets and leveraging them to further grow our business.”
Aphex BioCleanse Systems Announces Assignment of CAS Registry Numbers® and Trademarks in EU and UK for Proprietary Hy-IQ® Water
PITTSFORD, N.Y., June 17, 2021 (GLOBE NEWSWIRE) — via NewMediaWire — Aphex BioCleanse Systems, Inc. ( OTCPK: SNST ) (“Aphex” or “the Company”), a sanitization solutions company focused on the development and distribution of non-toxic and water-based sanitization and disinfection products trademarked as Hy-IQ® Water, announced today that the Company has been assigned two CAS Registry Numbers® for its proprietary Hy-IQ® Water. The Company also recently received trademarks for several products that utilize the power of Hy-IQ® Water in the European Union (EU) and the United Kingdom (UK).
The ability to list Hy-IQ on a product label will focus the company’s intellectual property directly where it matters. Aphex’s recent trademarks in the EU and the UK will further underpin the use of Hy-IQ® Water with recognition throughout most of Europe.
“As we continue to work toward FDA, USDA and EPA approvals of Hy-IQ® Water, the assignment of specific CAS Numbers puts us one step closer to achieving those goals,” said Aphex President and CEO David J. Weaver. “Additionally, as we look to the global market, our trademarks in the UK and the EU make it clear that what we have created is globally unique and holds the ability to revolutionize the entire world’s sanitization standards.”
The Company is currently in the multi-step process of gaining several federal approvals with the Environmental Protection Agency (EPA), United States Department of Agriculture (USDA) and U.S. Food and Drug Administration (FDA). Specifically, approval from the FDA would make Aphex the manufacturer of the only FDA-approved hand sanitizer.
Hy-IQ® Water is the Company’s proprietary, nontoxic, nonflammable, and alcohol-free active ingredient. The ingredient is highly versatile and has been proven effective in independent laboratory testing against an extensive list of pathogens, including human coronavirus, swine flu, E. coli, influenza, Candida auris, Candida albicans and MRSA.
FDA Statement
The statements referenced documents have not been evaluated or approved by the FDA. The products and statements referenced in this document are not intended to diagnose, treat, cure, or prevent any disease.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements are based on the current plans and expectations of management and are subject to a number of uncertainties and risks that could significantly affect the company’s current plans and expectations, as well as future results of operations and financial condition. The company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Aphex is a client of Institutional Analyst, and Revelers.IO Media please reports for disclosure and disclaimer details.