“Proof of Concept. We take note that Eli Lilly (LLY-Not Rated) reported yesterday’s
positive interim results from a placebo-controlled trial of a similar compound to STI1499. We believe the Sorrento antibody could be more potent and manufactured more
easily at a larger scale.” Dawson James Securities (see report below).
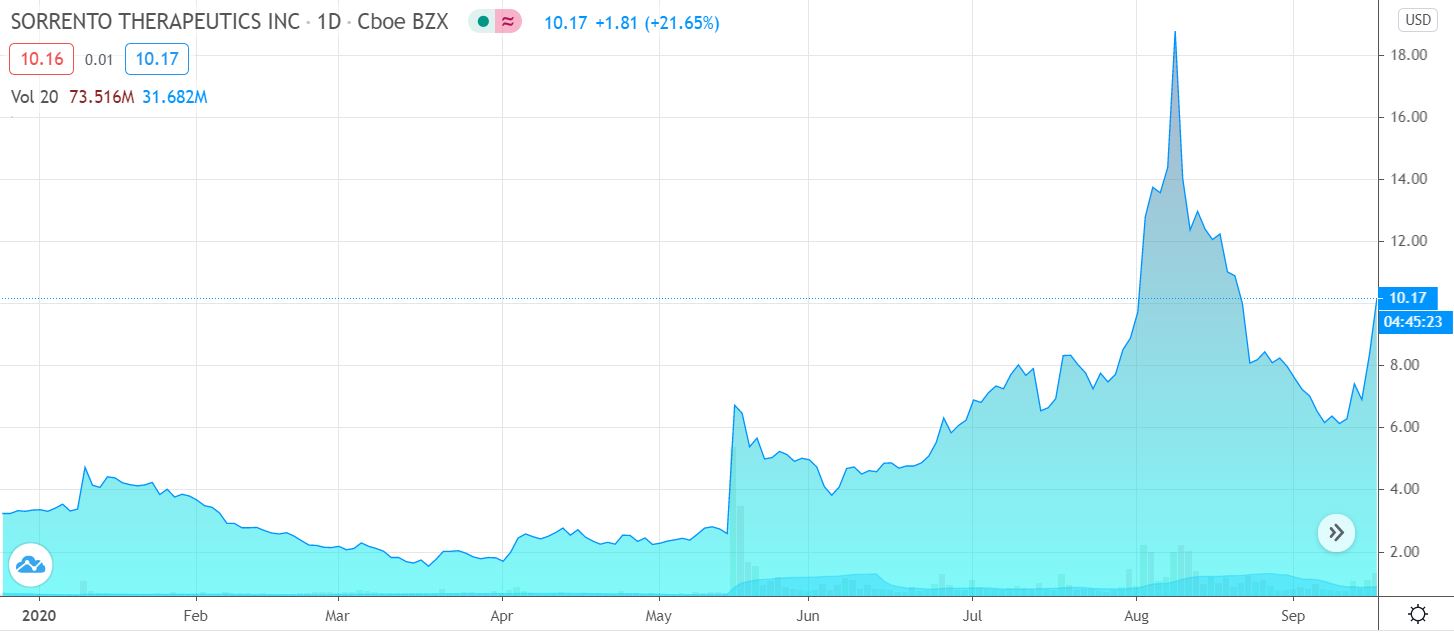
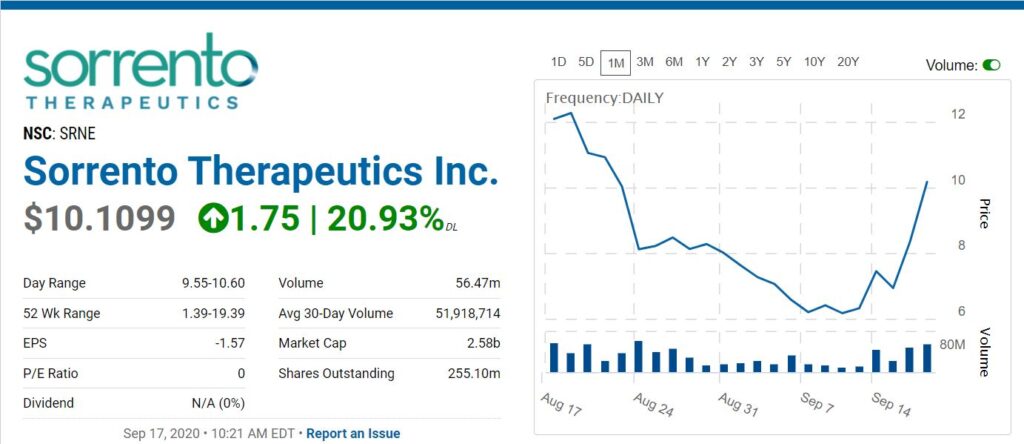
Why Sorrento Therapeutics Stock Is Ripping Higher Today (MF)
Sorrento Receives US FDA Clearance to Proceed With Phase 1 Clinical Trial of STI-1499 (COVI-GUARD) Neutralizing Antibody in COVID-19 Positive Patients
- Phase 1 clinical trial for STI-1499 (COVI-GUARD™) in hospitalized COVID-19 patients has received FDA notice that it may proceed with patient enrollment.
- The initial trial is expected to enroll rapidly and is expected to be followed by large trials targeting a potential Emergency Use Authorization (EUA) submission as early as before the end of this year.
- Sorrento has initiated cGMP manufacturing to produce 50,000 doses in anticipation of a potential EUA.
SAN DIEGO, Sept. 16, 2020 (GLOBE NEWSWIRE) — Sorrento Therapeutics, Inc. (Nasdaq: SRNE, “Sorrento”) announced today that it received a study may proceed letter from the FDA for its phase 1 clinical trial for COVI-GUARD (STI-1499) in hospitalized COVID-19 patients.
As Sorrento previously announced, in preclinical studies, STI-1499 demonstrated 100% in vitro neutralizing effect against SARS-CoV-2, preventing infection of healthy cells in such preclinical in vitro studies.
STI-1499 was further evaluated in preclinical studies using multiple strains of SARS-CoV-2, including the highly contagious D614G variant. In these preclinical studies, the antibody has been 100% effective against the highly contagious D614G variant strain at similar doses to those observed in experiments with the USA-WA1/2020 strain.