Prevent Montezuma’s Revenge BEFORE it starts! Immuron’s direct to consumer product Travelan® is a huge hit with international travelers’ with over $1 million of their magic pills being snapped up in the first half of fiscal 2020. North American (x-US) sales were up 98% year over year. Travelers say “it really, really works!”
Immuron is a publicly listed Australian bio-pharmaceutical company dual-listed in the US under the symbol IMRN.
Once approved by the FDA, the company believes ‘IMM-124E‘ would be the first and only FDA approved prophylactic effective against acute infectious diarrhea.
Overall, diarrhea leads to an estimated 1.5 billion episodes a year globally, killing about 2.2 million people, mostly children in developing countries.
Immuron is also advancing trials (where our interest lies) of solutions for deadly bacterial strains such as Campylobacter, Cholera, e.Coli, Shigella, and C.diff.
C.diff caused almost half a million infections among patients in the United States in a single year, according to a study released today by the Centers for Disease Control and Prevention (CDC). Approximately 29,000 patients died within 30 days of the initial diagnosis of C. difficile.
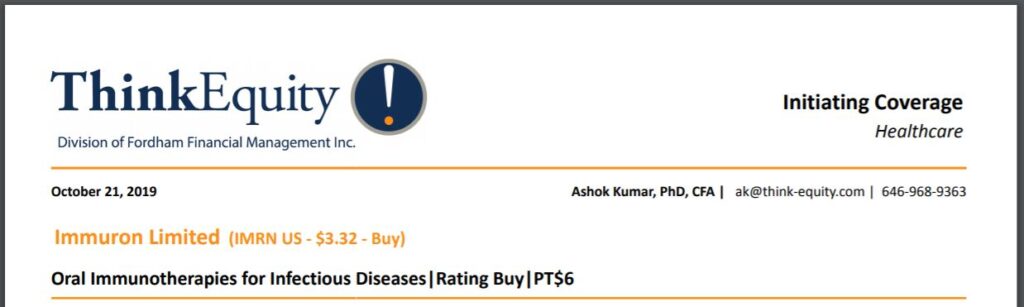
In July 2019, the company successfully completed a USD $1.24 million public offering of American Depository Shares (ADS) via ThinkEquity, a division of Fordham Financial Management, were the underwriters for the financing.
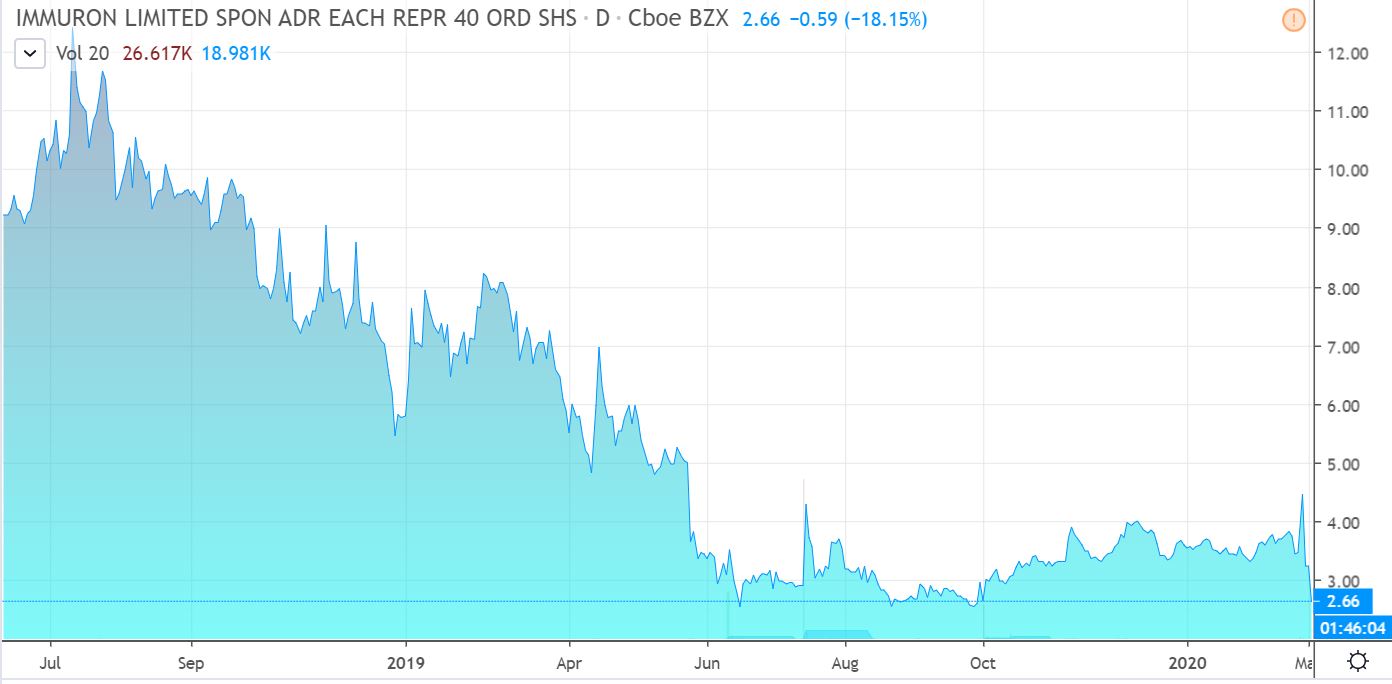
Grossly undervalued in our opinion, with a market capitalization just shy of $10 million at current prices. ThinkEquity issued coverage in October with a price target of $6.00 – or better than a double.
Immuron Provides Update on First Half of FY20 Results
Key Highlights:
- Strong continued growth of Travelan® sales reported in all markets
- Global sales reached USD $1.1 million (AUD $1.68 million) for first half FY 2020 up 55% from first half FY 2019
- North American Travelan® sales up by 98% YoY in the first half of FY20
- IMM-124E/Travelan® US registration strategy – Pre-IND meeting completed with the U.S. FDA
- USD $3.7 (AUD $5.5) million non-dilutive funding approved by US Department of Defense to develop and clinically evaluate a new therapeutic targeting Campylobacter and ETEC
- FDA strategy updated for clinical development of IMM-529
- U.S. Department of Defense Travelan® Shigellosis animal study results reported
- Three new Shigella drug product candidates commence preclinical evaluation by WRAIR
- Research and development tax concession refund paid
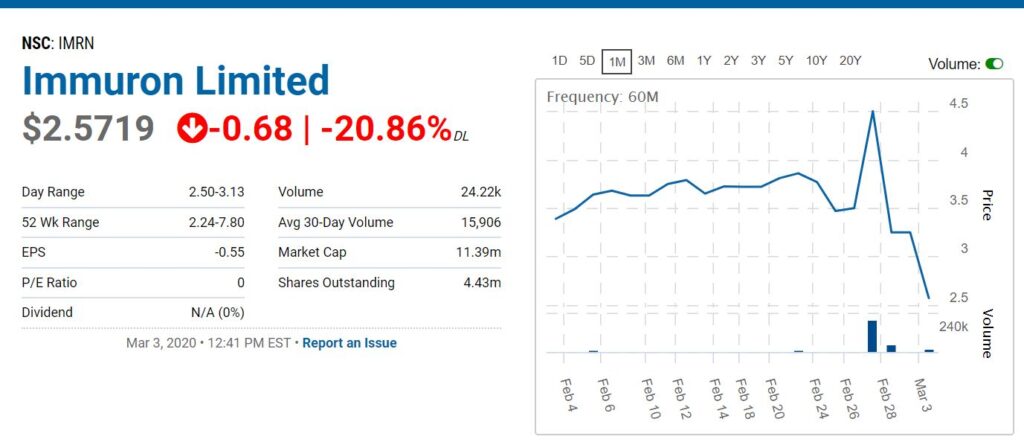
MELBOURNE, Australia, March 03, 2020 (GLOBE NEWSWIRE) — Immuron Limited (ASX: IMC; NASDQ: IMRN), an Australian biopharmaceutical company focused on developing and commercializing oral immunotherapeutics for the treatment of gut mediated diseases, today announced an update of its results for the first half of fiscal year 2020 ended on December 31, 2019.
Travelan® enjoys continued high growth in all markets
Immuron experienced robust gross sales growth in the US, Canada and Australia throughout the first half of FY20, with global sales reaching USD $1.1M* (AUD $1.68M*) during the 6-month period.
North America sales of Travelan® were up 98% YoY for the first half of FY20 from the first half of FY 2019, spurred on by the launch of Travelan® in Canadian pharmacies in June 2019 and also by robust growth in online Amazon sales within the US. Passport Health, the USA’s largest travel medicine provider, also contributed to the strong result, with Travelan® sales rising by 27% from first half FY 2019 within the Passport Health network of clinics. A series of podcasts on the “Not old, better” network assisted in raising consumer awareness of Travelan® in the US.
In Australia, Immuron sales reached USD $622K* (AUD $954K*) for the first half FY20, displaying a 33% YoY growth rate. Travelan® strengthened its presence in Australian pharmacies with in-store promotional material and TV advertising with Chemist Warehouse. Immuron’s participation in Medical Practitioner conferences also contributed to increased awareness of Travelan® within the medical community.
FDA registration for clinical development of IMM-124E/Travelan® to prevent travelers’ diarrhea underway
In April 2019, Immuron announced plans to pursue clinical development of IMM-124E through a formal FDA registration pathway as a drug to prevent travelers’ diarrhea (TD). This is an important strategic initiative towards enhancing commercialisation of the IMM-124E/Travelan® franchise. On November 21, 2019, the company announced that it had completed a Pre-IND meeting with the U.S. Food and Drug Administration (FDA) regarding its investigational drug IMM-124E to treat travelers’ diarrhea (TD). Following the FDA’s guidance and feedback, the company announced plans to file an investigational new drug (IND) application for IMM-124E, and to conduct a Phase 3 trial of IMM-124E to prevent TD in individuals traveling to areas endemic for TD. Immuron believes that success with the clinical trial, followed by a BLA filing with the FDA, and successful FDA approval of IMM-124E to specifically prevent travelers’ diarrhea could lead to substantial increases in sales of an FDA-approved drug to treat travelers’ diarrhea.
Once shown to work, and successfully approved, we believe IMM-124E would be the first and only FDA approved prophylactic effective against acute infectious diarrhea. Overall, diarrhea leads to an estimated 1.5 billion episodes a year globally, killing about 2.2 million people, mostly children in developing countries.
Naval Medical Research Center (NMRC) grant funded to develop and clinically evaluate new therapeutic against Campylobacter
On October 2, 2019, Immuron announced funding by the U.S. Department of Defense (DoD) of a new research agreement with America’s Naval Medical Research Centre (NMRC), a research arm of the DoD, located in Silver Spring, Maryland, to develop a combined Campylobacter and enterotoxigenic E. coli (ETEC)-specific drug candidate for clinical evaluation. Under this agreement, Immuron and NMRC will be collaborating on the manufacture and evaluation of the new product candidate designed to protect against travelers’ diarrhea caused by Campylobacter and ETEC pathogens. The protective efficacy of the candidate product will be evaluated utilizing two controlled human infection-model clinical trials, with one trial focusing on the ability of the hyperimmune product to protect volunteers against moderate to severe campylobacteriosis, and the second trial focusing on preventing ETEC-specific diarrhea.
Positive results for U.S. DoD study on Travelan® and Vibrio cholera
A prior study conducted during the previous year showed Travelan’s immuno-reactivity to infectious Vibrio cholera strains from Southeast Asia. The U.S. Department of Defense sponsored the project conducted at the Bangkok laboratory of the Walter Reed Army Institute of Research. Clinical isolates were collected from infected U.S. personnel stationed in Bangladesh, Cambodia, and Thailand. The new study found Travelan’s polyclonal antibodies were reactive to all 71 clinical isolates from infected participants. The 71 add on to the 180 isolates of Campylobacter spp, ETEC, and Shigella spp from the earlier 2018 study. The results, along with findings from primate shigellosis studies, point towards Travelan as a potentially effective immuno-prophylactic for travelers’ diarrhea caused by these pathogens.
American depository shares (ADS) capital raise completed
In July 2019, the company successfully completed a USD $1.24 million (AUD $1.9 million) public offering of American Depository Shares (ADS). Immuron issued 339,130 ADSs, equivalent to 13,565,200 fully paid ordinary shares. The proceeds will go towards clinical development of our therapeutic drug candidates, as well as for working capital.
ThinkEquity, a division of Fordham Financial Management, were the underwriters for the financing.
IMM-124E trial in SAH patients leads to decision to discontinue further development of IMM-124E in this and similar indications
In August 2019, the results from a Phase II clinical study in patients with severe alcoholic hepatitis (SAH), conducted under FDA IND #015675 and funded by the National Institute of Alcohol Abuse and Alcoholism (NIAAA), were released. The primary objective of the study was to evaluate the safety and efficacy of IMM-124E at two oral dosage levels as compared with a placebo in patients with SAH and with all patients also being treated with steroids. The data showed that IMM-124E did not reduce circulating lipopolysaccharide levels, mortality or have an impact on MELD score in the study population. Further clinical development of IMM-124E to treat SAH and similar indications has been discontinued.
IMM-529 trial in patients with C. difficile infection (CDI)
In March 2019, Immuron provided an update regarding the status of the IMM-529 clinical trial in patients with CDI, along with a refocusing of its efforts to develop IMM-529. The Phase I/IIa clinical trial of IMM-529 in patients with CDI initiated at the end of 2017 at two clinics in Israel exhibited poor patient enrollment, with only nine patients being randomized into a study planned to enroll 60 patients. Immuron decided to close these sites and to focus further development of IMM-529 to treat CDI patients through a formal filing of an IND with FDA, and to develop a new plan for development of the drug candidate to treat patients subject to recurrent disease, a major unmet medical need in the treatment of patients suffering with C. difficile infections. The company plans to file a Type B meeting request with FDA to explore further development of IMM-529.
U.S. Department of Defense’s Travelan Shigellosis animal study results reported
In June 2019, we updated the market on the Shigella research program with the Walter Reed Army Institute of Research (WRAIR). Shigella is the bacterium responsible for the onset of bacillary dysentery, and a major concern for armed forces personnel located in high risk areas for this disease throughout the world. The study results demonstrated that animals with severe inflammation in the gastrointestinal tract and high inflammatory cytokines in fecal samples were associated with severe bacillary dysentery, and that those animals treated with prophylactic administration of Travelan significantly reduced the inflammatory response.
Preclinical Evaluation of three new Shigella drug products
In the same June 2019 announcement, we reported the completion of the manufacture of three new Shigella-specific therapeutic products using proprietary vaccines developed by WRAIR. The immune reactivity of the three hyper-immune Shigella-specific bovine colostrum products have been assessed by WRAIR using ELISA and Western Blot analysis. The antibodies in these products were shown to react with the specific antigens present in the vaccines and were also reactive to four different clinical isolates of Shigella (S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei). These three Immuron Shigella-specific therapeutic products are now undergoing further evaluation in WRAIR’s preclinical models of shigellosis, with results expected to be reported throughout this year.
Research and development tax concession refund paid
The Australian government has paid Immuron a cash refund of USD $345,560 (AUD $530,000) as part of its Research and Development Income Tax Concession program.
Immuron CEO, Dr. Gary S. Jacob, said “We are pleased to report continued sales momentum globally for Travelan® as consumer awareness continues to grow. Our work toward FDA registration remains ongoing and we believe it provides a further boost to our long-term sales potential. Cash flow from increasing Travelan® sales, combined with our successful ADS offering and non-dilutive tax concession R&D refund, provide a solid foundation to move our development programs with IMM-529 and IMM-124E forward. We will provide further updates as these and other in-house programs progress.”
*Unaudited gross revenue
| COMPANY CONTACT: Gary S. Jacob, Ph.D. Chief Executive Officer Ph: +61 (0)3 9824 5254 [email protected] | AUS INVESTOR RELATIONS: Peter Taylor NWR Communications Ph: +61 (0)4 1203 6231 [email protected] | USA INVESTOR RELATIONS: Dave Gentry – CEO RedChip Companies, Inc. US Ph: +1 (407) 491 4498 [email protected] |
About Travelan®
Travelan® is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea. Travelan® is a highly purified tabletized preparation of hyper immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing bacteria and prevent colonization and the pathology associated with travelers’ diarrhea. In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Travelers’ Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product (NPN 80046016) and is indicated to reduce the risk of Travelers’ Diarrhea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection.
About Travelers’ diarrhea
Travelers’ diarrhea is a gastrointestinal infection with symptoms that include loose, watery (and occasionally bloody) stools, abdominal cramping, bloating, and fever, Enteropathogenic bacteria are responsible for most cases, with enterotoxigenic Escherichia coli (ETEC) playing a dominant causative role. Campylobacter spp. are also responsible for a significant proportion of cases. The more serious infections with Salmonella spp. the bacillary dysentery organisms belonging to Shigella spp. and Vibrio spp. (the causative agent of cholera) are often confused with travelers’ diarrhea as they may be contracted while travelling and initial symptoms are often indistinguishable.
About Immuron
Immuron Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of inflammatory mediated and infectious diseases. Immuron has a novel and safe technology platform with one commercial asset generating revenue. In Australia, Travelan® is a listed medicine on the Australian Register of Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Travellers’ Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licenced natural health product (NPN 80046016) and is indicated to reduce the risk of Travellers’ Diarrhea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection in accordance with section 403 (r)(6) of the Federal Drug Administration (FDA). Immuron’s lead clinical candidate, IMM-124E, is presently in Phase II trials in Severe Alcoholic Hepatitis (SAH) and Pediatric Nonalcoholic Fatty Liver Disease (NAFLD). The company now has plans to develop a U.S. registration dossier for IMM-124E for Travellers’ Diarrhea. Immuron’s second clinical-stage asset, IMM-529, targets Clostridium difficile Infections (CDI), and is in clinical trial development in CDI patients. These products together with the Company’s other preclinical immunotherapy pipeline products currently under development targeting immune-related and infectious diseases are anticipated to meet pressing needs in the global immunotherapy market.
For more information visit: http://www.immuron.com
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
#IMRN, #SUPERBUG,