SHORT TERM CHART
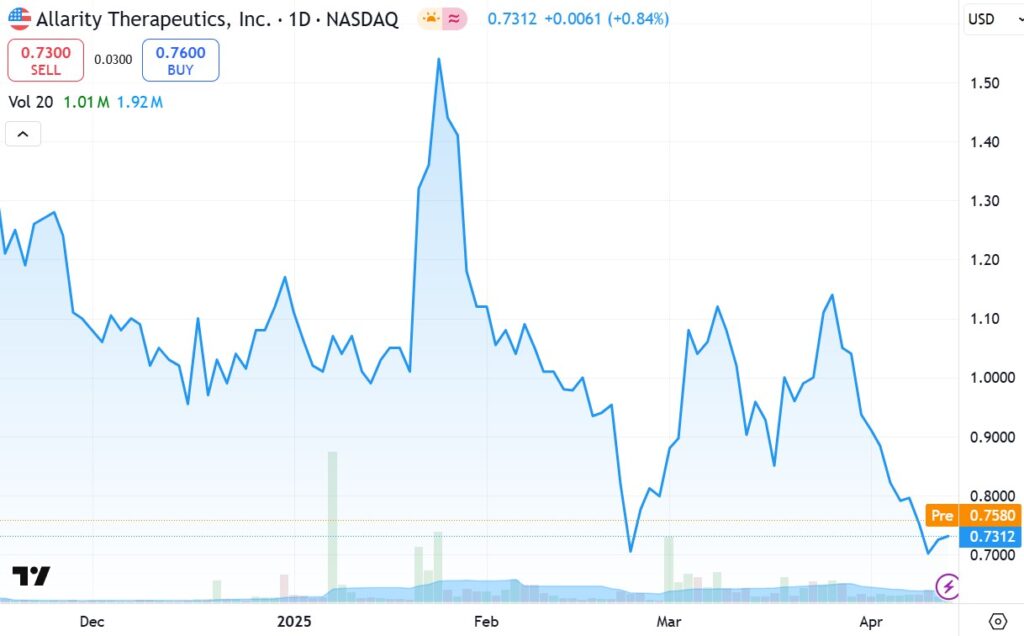
Adding to the Watch List at $0.70, for now, as a ‘technical’ play. Meaning the chart feels like, after a very rough six months, that we may be near a bottom. With a $12 million market cap, any good news could send the shares up 3-4-6 fold higher – or even more. Much, much more..
Looking at the above chart, one could say we are bargain hunting. A return to the February high would mean a double.
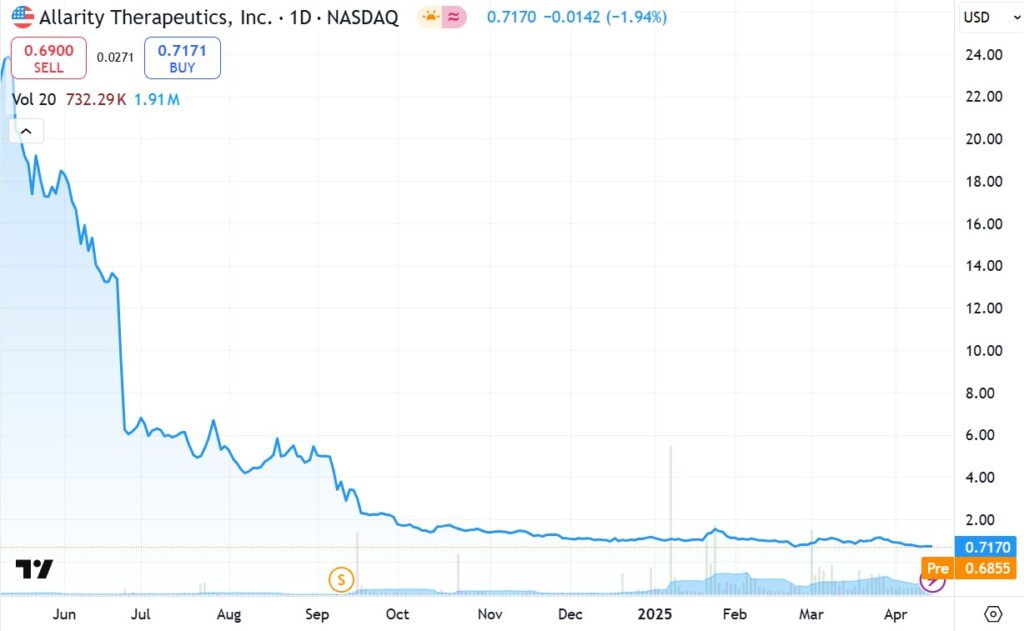
Looking at the below chart, one could say we are bottom fishing. A return to the one-year high of $24 would mean a gain of 3,300%. The likelihood of that? Depends on the news related to Stenoparib in combination with Temozolomide, for small cell lung cancer – which is heading into a Phase II trial.
And seperately Stenoparib, for Ovarian cancer. It is currently in a Phase II trial with a ‘new’ protocol. The new and improved protocol was designed with feedback from the FDA, their Institutional Review Board and their treating physicians!
ONE YEAR CHART
TWO YEAR CHART
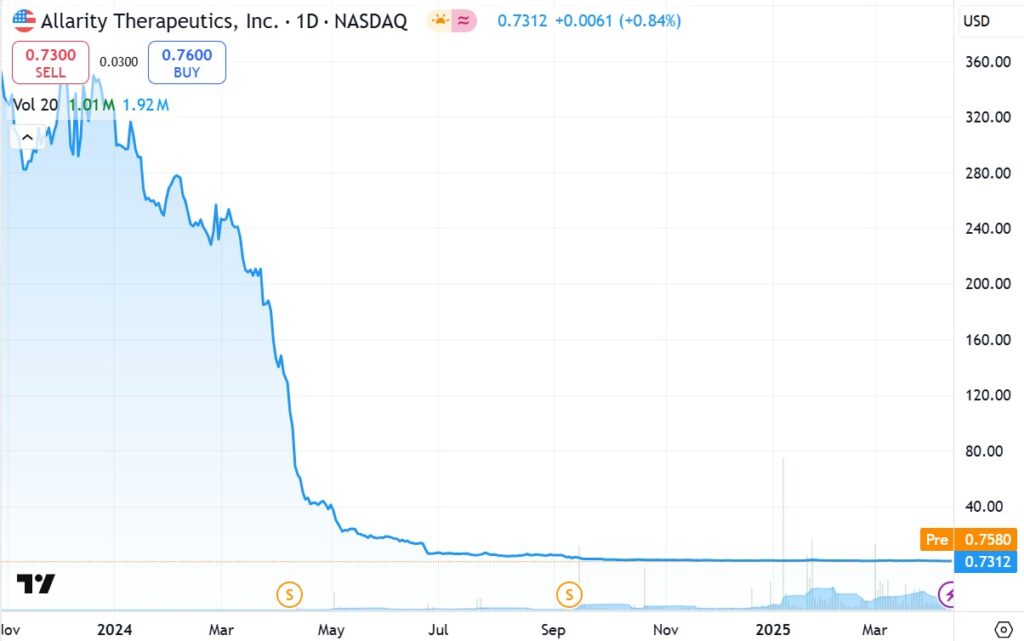
Looking at the next chart below, one could say we are abyss hunting. A term we’ve never used before. Note the shares were never actually at $11,000. They were ‘effectively’ at $11,000, the result of four reverse splits, in roughly three years.
THREE YEAR CHART
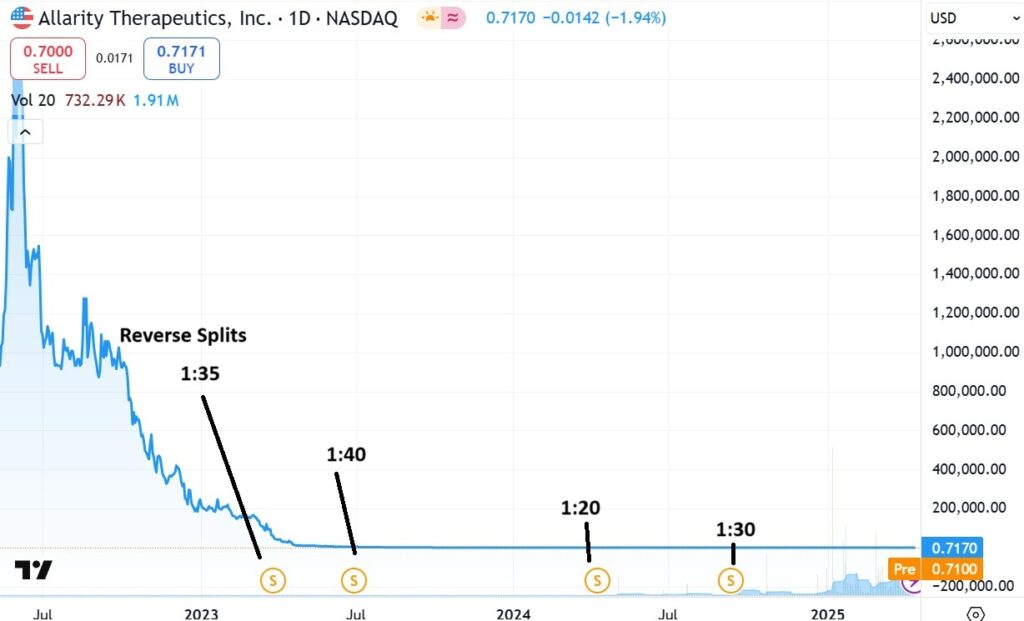
The odds of returning to the old high or investors recovering their intial stake? Zip, nada, zilch. But one investors problem, is anothers’ opportunity.
IF they were to get FDA approval (which we are not predicting) with only 17 million shares left outstanding after the reverse splits, we could make the case of a $500 million market cap or $29 a share. Their inhibitor addresses a $9 billion market.
So we are additionally adding Allarity to our Reverse Split Stock Watch List. The whole point of covering companies which have done a reverse is that are so few shares outstanding after the splits, even a ‘hint’ of good news, can send share prices 10-fold higher.
And $29 would be possible if either of their indications for Stenoparib gets approved by the FDA. BTW we never bet on the odds of FDA approval in biotechs. We instead bet on what the stock could do IF they get approval.
So is There a Chance of Good News on the Horizon?
Adding Allarity to the Watch List means we found something very interesting and that we are begining to study it. If we continue to like we we uncover, we’ll author and issue a detailed research report and contact the company, to see if we can be retained for ongoing news reporting and coverage.
WHAT WE’LL BE STUDYING
(No Wall Street Coverage)

“The Board’s decision to authorize a share repurchase program of up to $5 million reflects our confidence in the Company’s long-term vision, the clinical potential of stenoparib, and our ability to advance its development to provide a much-needed treatment option for women battling advanced ovarian cancer,” commented Allarity CEO Thomas Jensen.
March 2025: 2024 Financial Results and Provides a Business Update.
March 2025: Announces Final Settlement with the U.S. Securities and Exchange Commission
March 2025: Announces Board Authorization of $5 Million Share Repurchase Program (that woul reduce share cout to 12 million at these prices – aka stretching the rebound rubber bandaid.
*Feb 2025: New Phase 2 Protocol to Advance Stenoparib Toward FDA Approval in Advanced Ovarian Cancer Patients
July 2023: Allarity Therapeutics, Inc. Announces Pricing of $11 Million Public Offering
Cash and Cash Equivalents: $19 Million.
Short Interest (yum). 1.2 million shares!

DISCLAIMER
This update contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements provide the Company’s current expectations or forecasts of future events. The words “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements include, but are not limited to, statements related to the initiation and progress of the updated Phase 2 protocol for stenoparib in platinum-resistant ovarian cancer, the launch and conduct of the fully VA-funded Phase 2 trial evaluating stenoparib in combination with temozolomide for small cell lung cancer, the durability and regulatory potential of clinical benefit observed in ongoing studies, the Company’s strengthened financial position and expected ability to fund operations into 2027, potential market expansion supported by recent patent grants, and the resolution of regulatory and legal matters. Any forward-looking statements in this press release are based on management’s current expectations of future events and are subject to multiple risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the risk that the Company may not be able to secure sufficient capital to support its ongoing and planned clinical development activities, including the updated ovarian cancer protocol and the new VA-funded SCLC trial; the risk that observed clinical benefit, including durable responses and disease stability, may not be replicated in larger or later-stage studies; the risk that final trial data may differ materially from interim observations; the risk that stenoparib may not receive regulatory approval or, if approved, may not achieve commercial success; the potential for delays or challenges in patient enrollment, site activation, or data collection; the risk that the Company’s DRP® companion diagnostic may not be validated or approved for use with stenoparib; and broader operational risks related to market conditions, regulatory developments, or unforeseen external events that could affect the Company’s clinical execution or financial trajectory. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our Form 10-K annual report filed with the Securities and Exchange Commission (the “SEC”) on March 31, 2025, available at the SEC’s website at www.sec.gov, and as well as discussions of potential risks, uncertainties and other important factors in the Company’s subsequent filings with the SEC. All information in this press release is as of the date of the release, and the Company undertakes no duty to update this information unless required by law. Not a news reporting and coverage, client but we may like them to be in the future.












