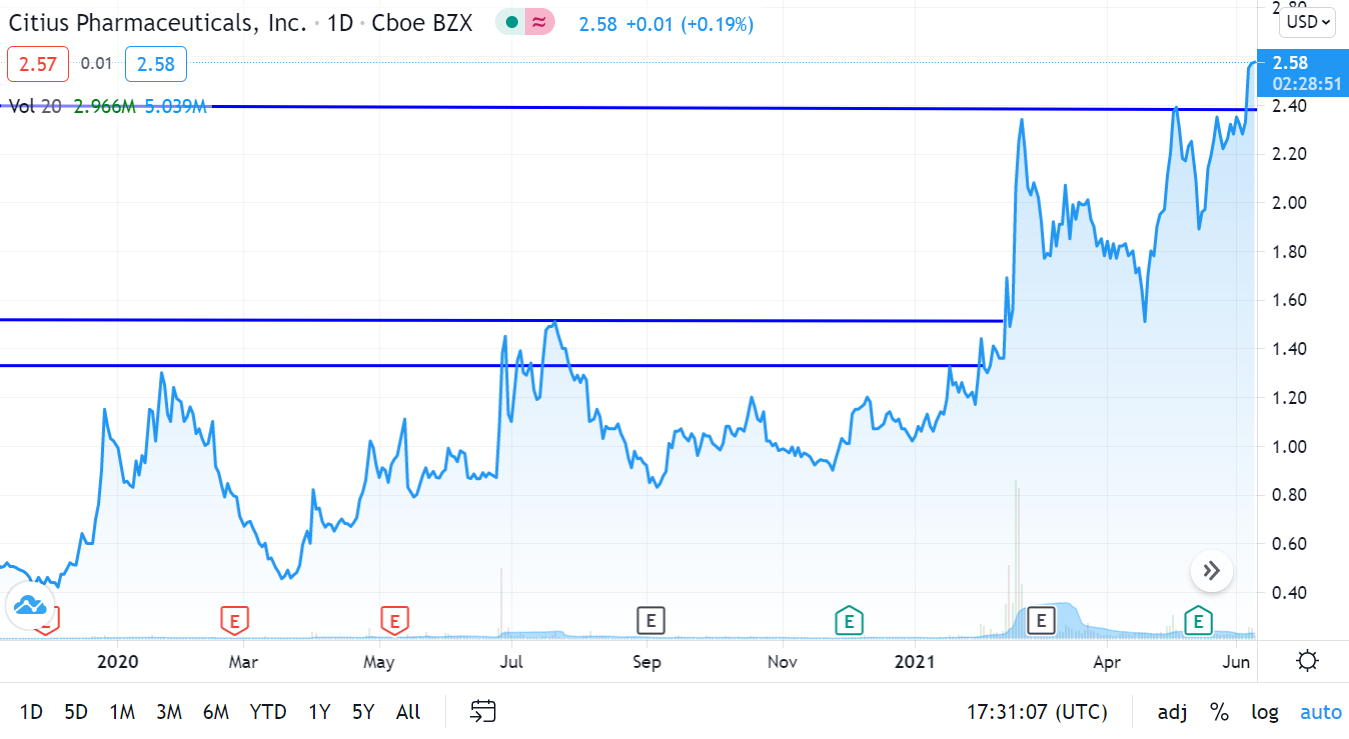
UPDATED CHART 6-15-21
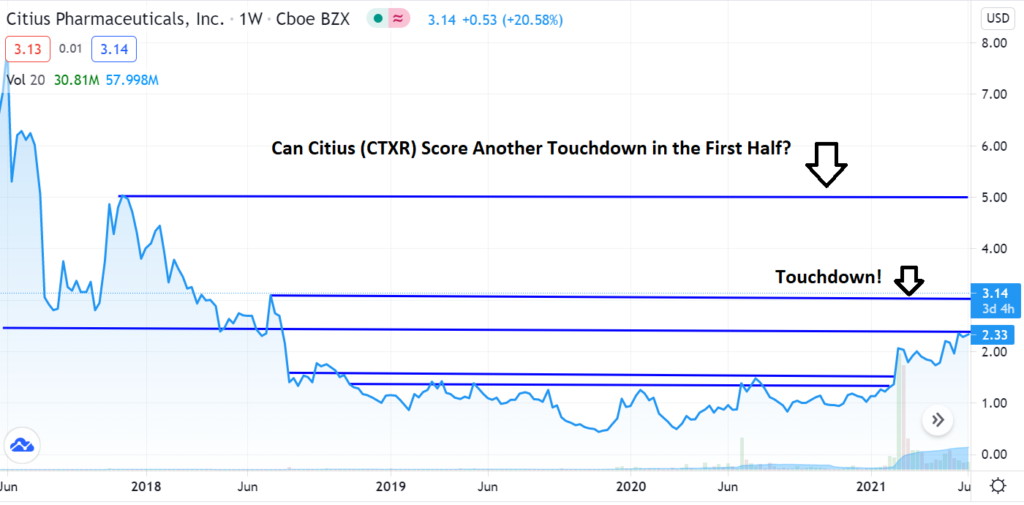
- Mino-Lok® Phase III, FDA News
- Russell 2000 Index News
- Technical Breakout News
We’re up 370% since adding it to the Biotech Stock Review Watch List in November of 2019, and things look better than ever.
Mino-Lok® Phase III, FDA News.
– Independent Data Monitoring Committee (DMC) to review Mino-Lok® safety, superiority, and futility data at upcoming meeting scheduled for June 29, 2021-
According to the Mino-Lok ® Phase 3 study protocol, the DMC is responsible for conducting interim analyses when 40%, 50% and 65% of the total number of anticipated events have been observed. The first two interim analyses were conducted by the DMC in 2019 and 2020, respectively. The next interim analysis meeting of the DMC will be held on June 29, 2021. At that time, the DMC will review unblinded study data and subsequently provide written recommendations to Citius within five business days.
The Mino-Lok ® Phase 3 pivotal superiority trial is a multi-center, randomized, open-label, blinded study to determine the efficacy and safety of Mino-Lok ® (MLT), a novel antibiotic lock therapy that combines minocycline with edetate disodium. The primary endpoint for this study is the time (in days following randomization) to a catheter failure event between randomization and TOC (Week 6) in the Intent-to-Treat (ITT) Population.
Approximately 144 subjects diagnosed with CRBSI/CLABSI and who meet all necessary criteria for the study are randomized in a 1:1 ratio to receive either Mino-Lok ® therapy or standard of care antibiotic lock therapy. To date, the Company has achieved more than 80% of the expected enrollment.
Russell 2000 Index News
Citius Pharmaceuticals announced that it is set to be added to the Russell 2000 ® Index at the conclusion of the Russell US Indexes annual reconstitution, effective at the opening of the U.S. equity markets on June 28, 2021.
Membership in the small-cap Russell 2000 ® Index, which remains in place for one year, is based on membership in the broad-market Russell 3000 Index. Citius stock will also be automatically added to the appropriate growth and value indexes.
Russell indexes are widely used by investment managers and institutional investors for index funds and as benchmarks for active investment strategies. Approximately $9 trillion in assets are benchmarked against Russell’s US indexes.
The Russell 1000 is a value-weighted stock index comprising the 1,000 largest firms by market capitalization; the Russell 2000 is a similar index that includes firms 1,001 to 3,000.
Top Russell 2000 Stocks With Highest Returns
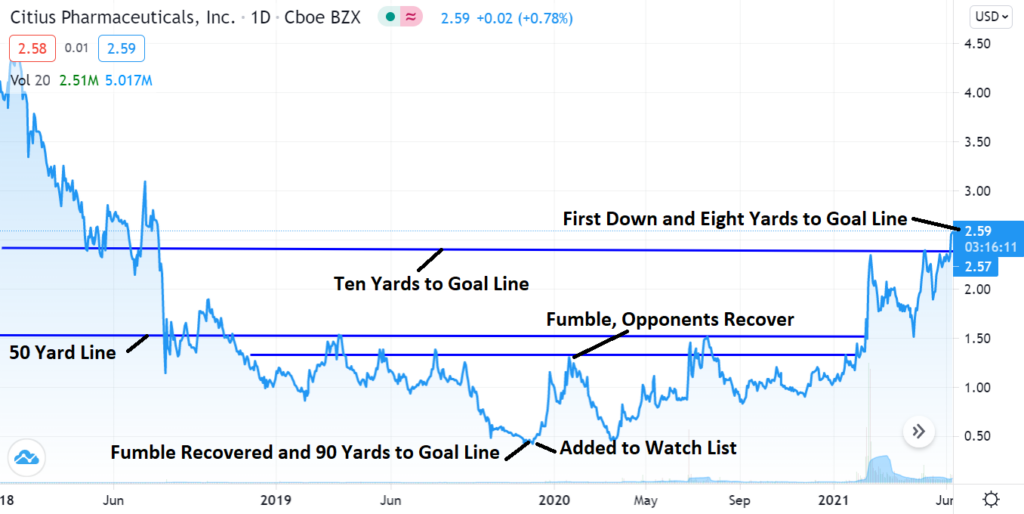
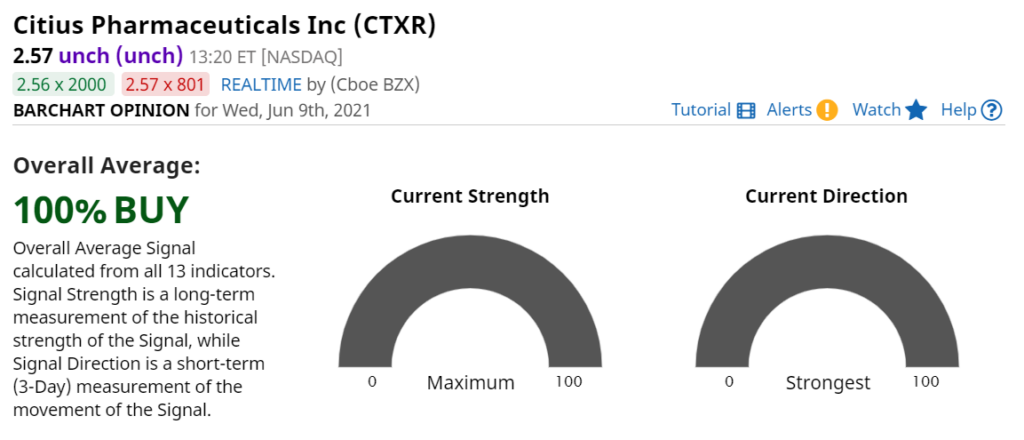
Technical Breakout News
A Sport Fans Take on the Technicals!
Disclaimer. Press releases linked to may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “plan,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: risks relating to becoming included and remaining included in the Russell 2000 index; our ability to successfully undertake and complete clinical trials and the results from those trials for our product candidates; risks relating to the results of research and development activities; uncertainties relating to preclinical and clinical testing; the early stage of products under development; the estimated markets for our product candidates and the acceptance thereof by any market; the ability of our product candidates to impact the quality of life of our target patient populations; our need for substantial additional funds; market and other conditions; risks related to our growth strategy; patent and intellectual property matters; our ability to attract, integrate, and retain key personnel; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; our dependence on third-party suppliers; our ability to procure cGMP commercial-scale supply; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further impacted by Covid-19. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”) filings which are available on the SEC’s website at www.sec.gov , including in our Annual Report on Form 10-K for the year ended September 30, 2020 , filed with the SEC on December 16, 2020 and updated by our subsequent filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law. Client, see reports for disclosure and disclaimer details.