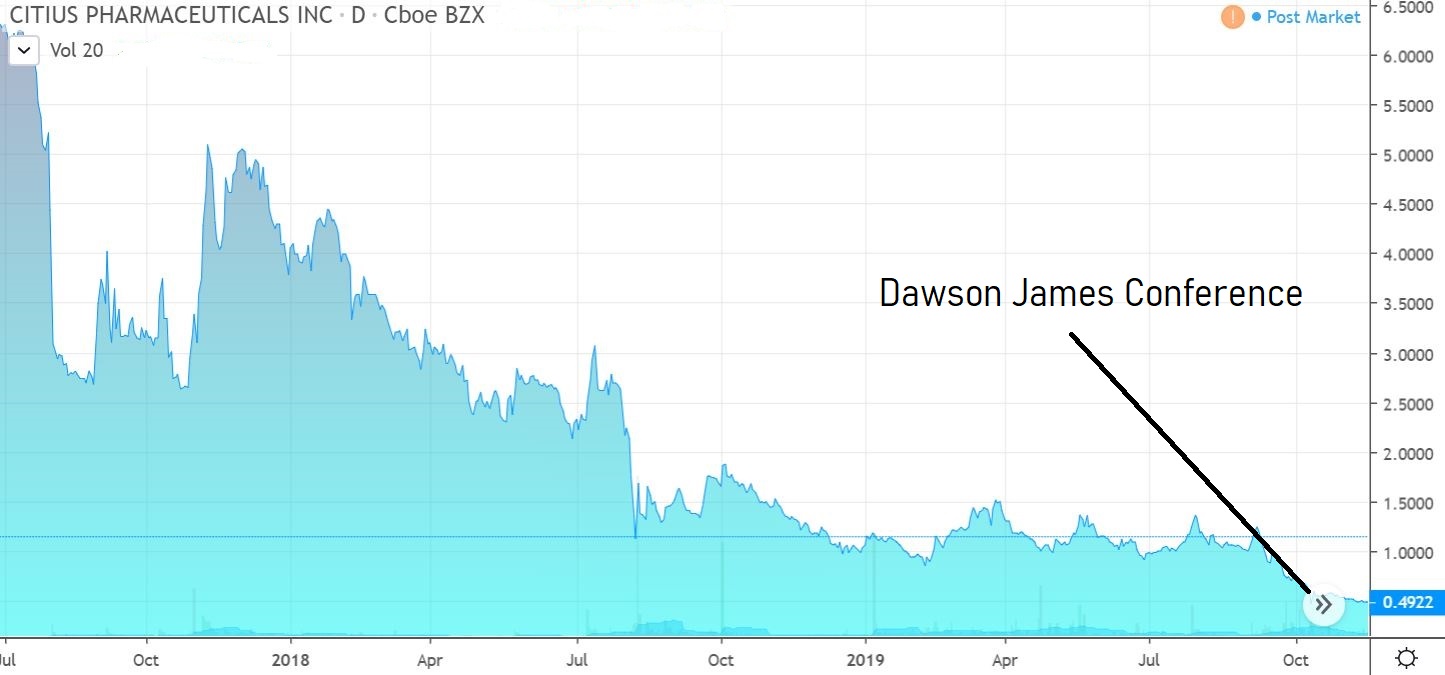
Best Presentation at Dawson James Conference.
We attended the Dawson James Conference on October 29th, which had over 20 medtech and biotech companies. The two most impressive presentations were from Fortress Biotech (FBIO) which we added to the Watch List at $1.73 and the heels of a $20 price target from Jason Kolbert and Citius Pharma (CTXR).
The Fortress plain explanation of their business model from Dr. Lindsay Rosenwald, something akin to an incubator, blew us away and went far beyond what you get from reading the regulatory filings or press releases. We searched for a replay but unfortunately couldn’t find one to share.
CITIUS PHARMA (CTXR) $0.55
What impressed us most about the Citius story was the tenacity of management as well as their vested interest, which is at a level quite like none we’ve ever seen before. They own 46% and have been buying.
We envision ten-bagger potential.
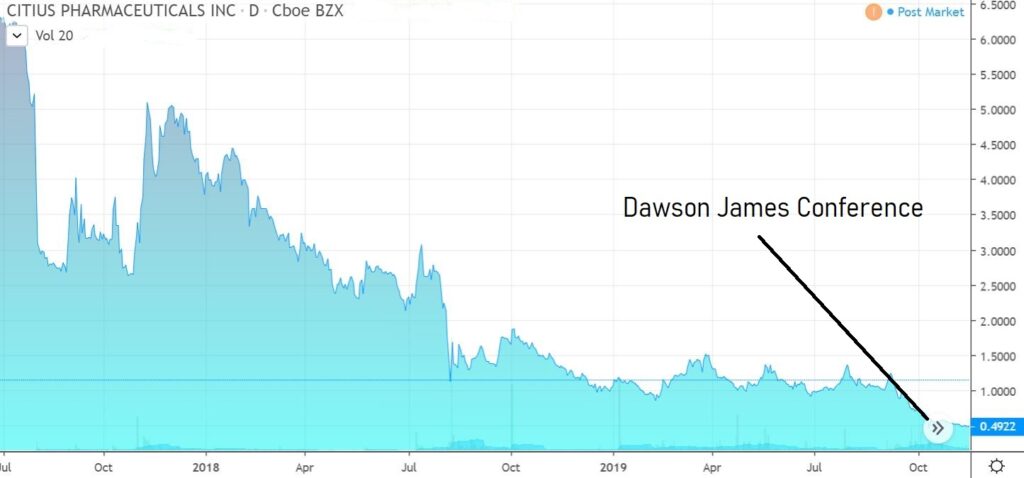
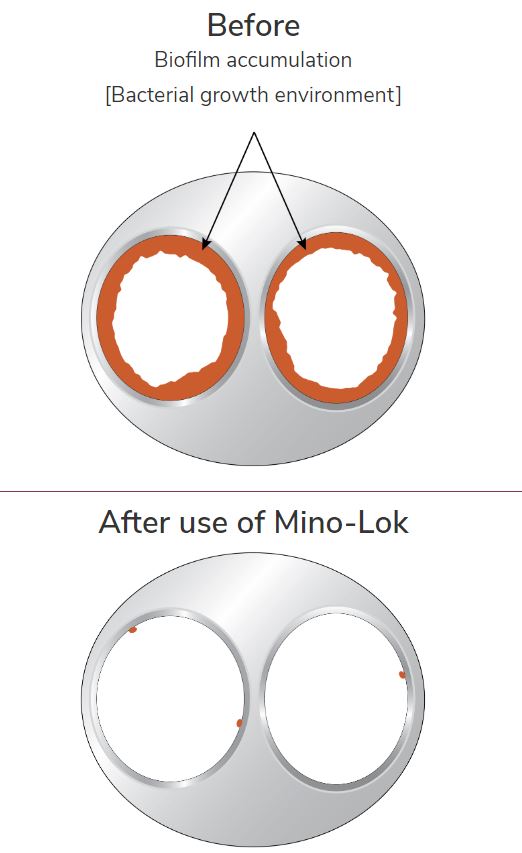
We will get into the product at a future date but add in the simplest terms, that it is a solution to ‘disinfect’ central venous catheters which have gotten infected. There are no competing products on the market and the current solution is the oftentimes life-threatening (15-20% complication rate) removal and replacement of the catheter.
The solution is called Mino-Lock.
The Company was founded in January 2007 as Citius Pharmaceuticals, LLC and began trading publicly in September 2014 through an agreement with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.). In March 2016 Citius acquired Leonard-Meron Biosciences (LMB) as a wholly-owned subsidiary. LMB was a pharmaceutical company focused on the development and commercialization of critical care products with a concentration on anti-infectives founded by Leonard Mazur and Myron Holubiak.
A light trader, volatility should be expected.
Yesterday they offered up a corporate update worth reading (bolding is ours).
Citius Pharmaceuticals Issues Corporate Update
Company provides updates on recent financing and advancements in its product candidates Mino-Lok®, Mino-Wrap™, and Halo-Lido
CRANFORD, N.J., Nov. 8, 2019 /PRNewswire/ — Citius Pharmaceuticals, Inc. (NASDAQ: CTXR) (“Citius” or the “Company”), a specialty pharmaceutical company focused on adjunctive cancer care and critical care drug products, today announced that the Company has issued its October 2019 Corporate Update Letter. The Letter highlights the progress of the Company’s product candidates Mino-Lok®, Mino-Wrap™, and Halo-Lido, along with details on its strategic goals to develop breakthrough technologies to improve and enhance the lives of patients.
Recent Company Highlights include:
- Closing on a recent $7 million capital raise to advance ongoing research
- A recent modification to the primary endpoint of the pivotal Phase III trial for Mino-Lok that substantially reduced the required trial sample size from 700 to approximately 144 subjects, significantly reducing the trial’s expense and accelerating its completion
- An expanded relationship with MD Anderson Cancer Center for a worldwide license for Mino-Wrap and the preparation for a pre-IND meeting with the FDA
- Reformulating the topical formulation (now Halo-Lido) for the treatment of hemorrhoids based on results from an initial Phase II study. A toxicology study will be initiated by year-end ahead of an expanded Phase II study to start in 2020
- Other corporate initiatives that include industry events and investor outreach
To view the Company’s Corporate Update Letter in its entirety, please visit: https://www.citiuspharma.com/wp-content/uploads/2019/11/CTXR-ShareholderLetter-Nov2019.pdf
About Citius Pharmaceuticals, Inc.
Citius is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products, with a focus on anti-infectives, cancer care, and unique prescription products that use innovative, patented, or proprietary formulations of previously-approved active pharmaceutical ingredients. We seek to achieve leading market positions by providing therapeutic products that address unmet medical needs. By using previously approved drugs with substantial safety and efficacy data, we seek to reduce the risks associated with pharmaceutical product development and regulatory requirements. Citius develops products that have intellectual property protection and competitive advantages to existing therapeutic approaches. For more information, please visit www.citiuspharma.com.
Safe Harbor
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are: risks associated with conducting our Phase 3 trial for Mino-Lok®, including completing patient enrollment, opening study sites and achieving the required number of catheter failure events; the estimated markets for our product candidates and the acceptance thereof by any market; our need for substantial additional funds; risks associated with developing Mino-Wrap™, including that preclinical results may not be predictive of clinical results and our ability to file an IND; risks related to our growth strategy; our ability to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; risks relating to the results of research and development activities; uncertainties relating to preclinical and clinical testing; the early stage of products under development; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to attract, integrate, and retain key personnel; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
Contact:
Andrew Scott
Vice President – Corporate Development
(O) 908-967-6677 x105
[email protected]