We’re now up 325% in just Under a Year. Last “big” News was Receiving a Priority Original Abbreviated New Drug Application, for KETARX™ (Ketamine) From the FDA.
Sparks expected by many to ‘fly’ in April of 2024 as the FDA assigned GDUFA goal date of April 29, 2024′ Locked and loaded as they say.
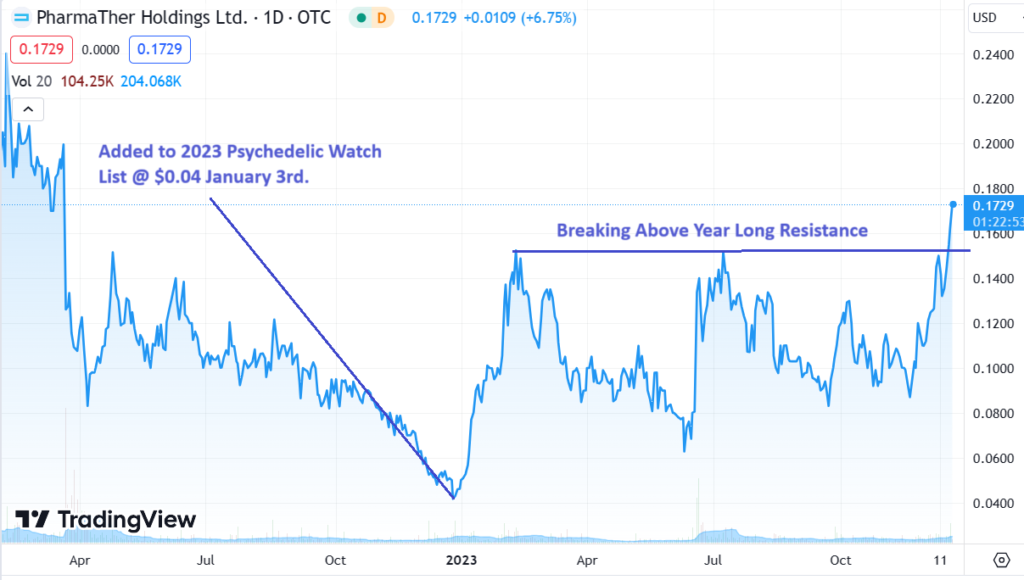
LONG TERM CHART
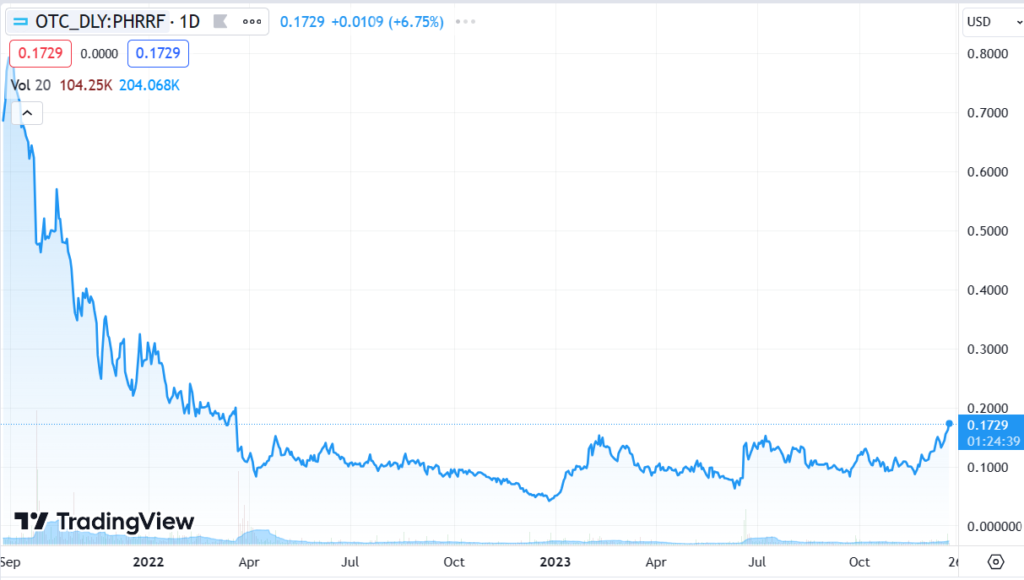
RELATED: JUNE 2022. $3.80 Price Target From H.C. Wainwright
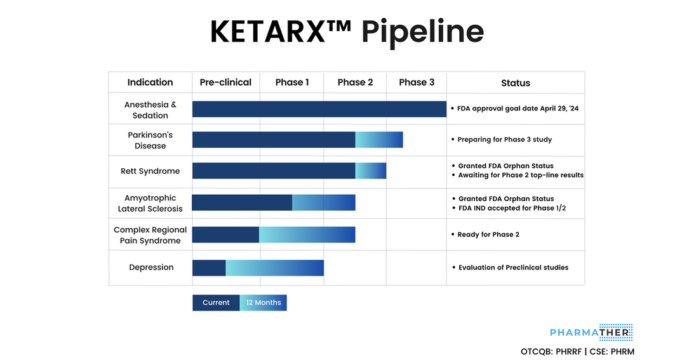
PharmaTher Announces FDA Acceptance, As a Priority Original Abbreviated New Drug Application, for KETARX™ (Ketamine)
FDA assigns GDUFA goal date of April 29, 2024
Milestone builds upon PharmaTher’s commitment toward unlocking the potential of ketamine for unmet medical needs
TORONTO, Sept. 27, 2023 (GLOBE NEWSWIRE) — PharmaTher Holdings Ltd. (the “Company” or “PharmaTher”) (OTCQB: PHRRF) (CSE: PHRM), a commercial-focused specialty pharmaceutical company, today announced the U.S. Food and Drug Administration (“FDA”) has accepted the Abbreviated New Drug Application (“ANDA”) for KETARX™ (racemic ketamine) to the Food and Drug Administration (“FDA”). The FDA assigned a Generic Drug User Fee Amendments of 2022 (“GDUFA”) goal date for this priority original ANDA of April 29, 2024. The Company anticipates the commercial launch of KETARX™ in the U.S. after that, followed by the pursuit of international approvals to support the growing global demand for ketamine.
“The acceptance of the ANDA for ketamine is a significant milestone for PharmaTher that brings us one step closer to becoming a global leader in ketamine for unmet medical needs,” said Fabio Chianelli, CEO of PharmaTher. “Ketamine has been on the FDA’s drug shortage list for over 5 years and its potential for mental health, neurological, and pain disorders continues to gain momentum and validation through published clinical research and real-world use cases.”
Subscribe to keep updated on PharmaTher.
Ketamine is an essential medicine used for anaesthesia and analgesia (pain relief) listed on the WHO Essential Medicines List. Ketamine has been on the FDA’s drug shortage list since February 2018. In Canada, ketamine has been classified as a Tier 3 drug shortage since February 2023, and Health Canada has approved ketamine for use as a sedative and painkiller in hospital settings.
Outside of the FDA and Health Canada approved indications, ketamine is also being administered in hospitals and clinics to treat various mental health, neurological and pain disorders. A recently published new peer-reviewed study on the real-world effectiveness of ketamine intravenous therapy demonstrated significant patient improvement for depression, anxiety and suicidal ideation.
PharmaTher’s priority is to commercialize KETARX™ in the U.S. through its recently announced partnership with Vitruvias Therapeutics, Inc., a leading U.S. based specialty generic pharmaceutical company. The Company expects to market various dosage forms of KETARX™, with the option to increase concentration and ready-to-administer applications for the U.S. and international markets.
About PharmaTher Holdings Ltd.
PharmaTher Holdings Ltd. (OTCQB: PHRRF) (CSE: PHRM) develops and commercializes specialty pharmaceuticals exhibiting growing adoption and permitting novel delivery methods to enhance patient outcomes. The Company’s lead product is KETARX™ (racemic ketamine) to fill the global unmet medical needs for anesthesia, sedation, pain, mental health, and neurological indications. Learn more at PharmaTher.com.
For more information about PharmaTher, please contact:
Fabio Chianelli
Chief Executive Officer
PharmaTher Holdings Ltd.
Tel: 1-888-846-3171
Email: info@pharmather.com
Website: www.pharmather.com
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
Cautionary Statement
This press release contains ‘forward-looking information’ within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words “could”, “would”, “intend”, “expect”, “believe”, “will”, “projected”, “estimated”, “potential”, “aim”, “may”, “plan”, “proposed”, “lead”, “toward”, “anticipate”, and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on PharmaTher Holdings Ltd. (the “Company”) current belief or assumptions as to the outcome and timing of such future events. Forward-looking information is based on reasonable assumptions that have been made by the Company at the date of the information and is subject to known and unknown risks, uncertainties, and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking information. Given these risks, uncertainties and assumptions, you should not unduly rely on these forward-looking statements. The forward-looking information contained in this press release is made as of the date hereof, and Company is not obligated to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities laws. The foregoing statements expressly qualify any forward-looking information contained herein. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption “Risk Factors” in Company’s management’s discussion and analysis for the three and nine months ended February 28, 2023 (“MD&A”), dated April 28, 2023, which is available on the Company’s profile at www.sedarplus.ca.
This news release does not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation or sale in any state, province, territory or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state, province, territory or jurisdiction.