American BriVision Announces New Patent Filed for Treatment of Major Depressive Disorder.
Phase II clinical study completed at Stanford University and five major medical centers in Taiwan.
The study concluded that PDC-1421 (the active ingredient) met the prespecified primary objective by demonstrating a highly significant 13.2-point reduction in depression as compared to a 9.2-point reduction for the placebo group. For the safety endpoint, both the low and high doses of PDC-1421 were safe and well tolerated with no serious adverse events.
(The global antidepressants market is expected to grow from $14.3 billion in 2019 to about $28.6 billion in 2020 as mental health issues are expected to surge due to the effects of the Covid-19 pandemic making an impact on the global economy. Biotech Stock Review: Research and Markets)
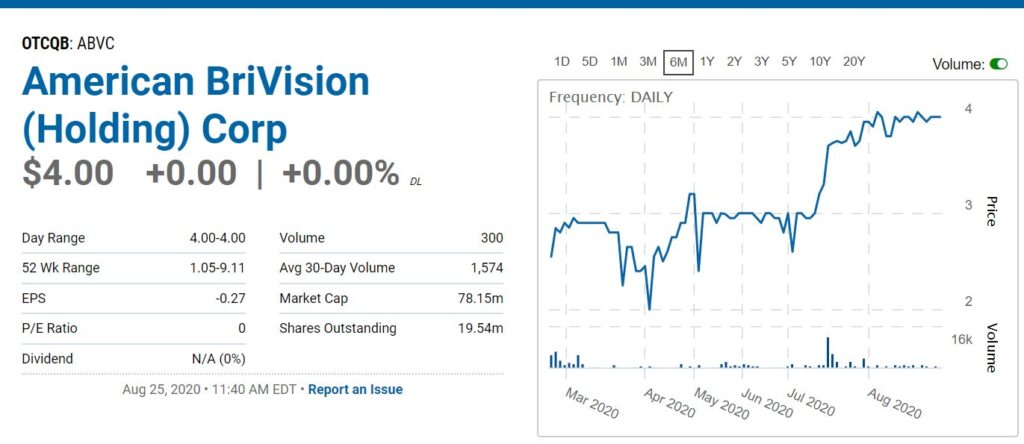
RELATED: Adding American BriVision (ABVC) $3.30 to Stocks We Expect Double List. $7.00 Price Potential.
FREMONT, CA, Aug. 24, 2020 American BriVision (Holding) Corporation (ABVC) , a clinical-stage biopharmaceutical company developing therapeutic solutions in oncology/hematology, CNS, and ophthalmology, today announced that on July 22, 2020, it had filed with the US Patent and Trademark Office (“USPTO”) a new patent for the treatment of Major Depressive Disorder (also known as clinical depression). It has been assigned serial number US 16/936,032.
The patent application, entitled “Polygala Extract for the Treatment of Major Depressive Disorder,” outlines a method for treating major depressive disorder by the patient’s oral administration of a composition containing Radix Polygalae (Polygala tenuifolia Willd) extract (PDC-1421).
ABV-1504 for Clinical Depression
Dr. Doong emphasized the importance of ABVC’s focus on botanical sourcing in drug development. “Our clinical trials continue to demonstrate that medicines derived from plants have significant therapeutic benefits with few – if any – side effects in treating serious medical conditions.”
“We believe this new patent, when granted, will greatly enhance our existing patent portfolio of Polygala tenuifolia Willd extract (PDC-1421),” said Dr. Howard Doong, ABVC chief executive officer. “It will serve to strengthen our global IP position for the treatment of major depressive disorder and provide product exclusivity through 2040.”
BIOTECH STOCK REVIEW NOTES
Some people have periods of depression separated by years, while others nearly always have symptoms present. Major depression is more severe and lasts longer than sadness, which is a normal part of life.
The most common time of onset is in a person’s 20s and 30s, with females affected about twice as often as males. Major depressive disorder affected approximately 163 million people (2% of the world’s population) in 2017.
The three most common treatments for depression are psychotherapy, medication, and electroconvulsive therapy. Psychotherapy is the treatment of choice (over medication) for people under 18. (Wiki).
TOP SELLING DRUGS
Every day, one out of six Americans takes a psychiatric medication, according to a 2017 study published in the Journal of the American Medical Association (JAMA). Twelve percent of these prescription drugs are antidepressants.
The global antidepressants market is expected to grow from $14.3 billion in 2019 to about $28.6 billion in 2020 as mental health issues are expected to surge due to the effects of the Covid-19 pandemic making an impact on the global economy. The market is expected to stabilize and reach $19 billion at a CAGR of 7.4% through 2023.
The antidepressants market has also surged during the covid-19 outbreak. The increasing number of cases and fatalities is affecting the mental health by elevating anxiety worldwide. People who are already living with mental health problems are experiencing increased stress levels over the Covid-19 outbreak. This has triggered the demand for antidepressant drugs.
TOP SELLERS
Serotonin reuptake inhibitors (SSRIs): Prozac, Celexa, Zoloft, Paxil, Lexapro.
Serotonin and norepinephrine reuptake inhibitors (SNRIs): Cymbalta, Effexor, Tramadol.
Atypical: Wellbutrin.
Prozac (Fluoxetine – now off patent) had sales of $2.8 billion at its peak and now is made by 13 manufacturers. Cymbalta had sales of $4.9 billion before losing its patent. We are researching the overall market size now.
About ABV-1504 for Clinical Depression
The World Health Organization has reported that more than 300 million people worldwide and of all ages suffer from major depressive disorder (MDD), also known as clinical depression. Figures from 2017 suggest that MDD affects over 17 million adults in the United States and is the leading cause of disability for ages 15-44.
In 2019, ABVC issued a completed Phase II clinical study report (CSR) under U.S. Food and Drug Administration (FDA) and Taiwan FDA (TFDA) procedures for ABV-1504 MDD medication.
PDC-1421 is the active pharmaceutical ingredient of ABV-1504. The study was conducted by Stanford University and five major medical centers in Taiwan and concluded that PDC-1421 met the prespecified primary objective by demonstrating a highly significant 13.2-point reduction in depression as compared to a 9.2-point reduction for the placebo group. For the safety endpoint, both the low and high doses of PDC-1421 were safe and well tolerated with no serious adverse events.
The Phase II results are expected to lead to Phase III and NDA-related plans and an eventual road map for market launch.
Client, see report for disclosure and disclaimer details.












