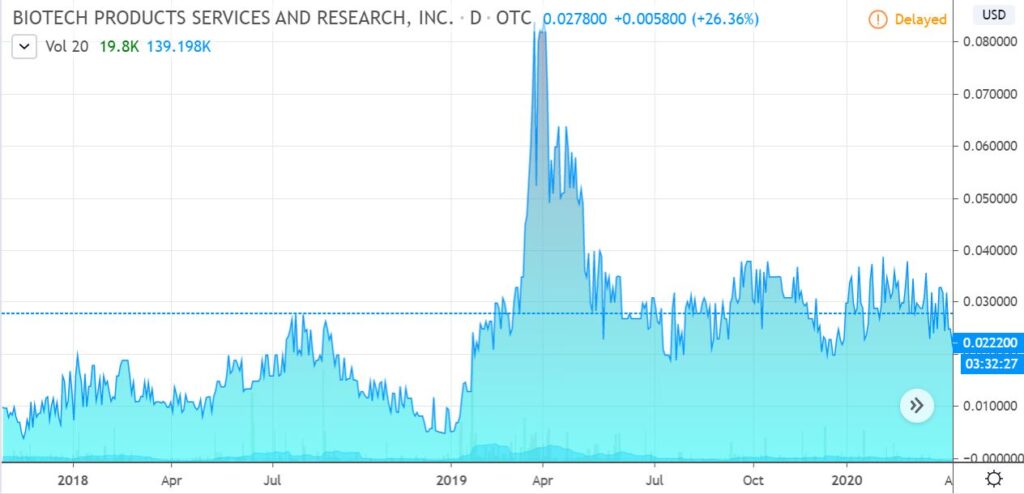
Organicell (BBSR). New client, studying now. Thinly, thinly traded and expectedly volatile on an intra-day basis. Like what we’ve read so far about their “Nano-Particle Therapeutic Platform” Organicell™ Flow which offers multiple uses, including for regenerative medicine.
Fantastic advisory board, something you wouldn’t expect to see with a $0.02 stock! Plenty of ‘issues’ but they are in the process of cleaning up*. Transfer agent verified 550 million shares outstanding, or a market cap of $12 million. The product is being sold and distributed to medical clinics and facilities.
Heavy on science, light on news for now. But not after we get started! Could be years of entertaining news ahead.
Undiscovered for Years, Are Things About to Change?
Sign Up For Organicell News Here
Organicell Regenerative Medicine Inc. Provides Update On Operations and Financial Reporting Status
MIAMI, Jan. 21, 2020 (GLOBE NEWSWIRE) — Organicell Regenerative Medicine Inc. (OTCPK:BPSR) (the “Company”) is pleased to provide shareholders and the investment community with an update on operations since its filing on November 1, 2018 of the Company’s Annual Report on Form filing of Form 10-K for the year ended October 31, 2017, as well as the status of becoming fully compliant with SEC reporting obligations.
The Company is diligently working to complete its Quarterly Reports on Form 10-Q for the quarters ended January 31, 2018, April 30, 2018 and July 31, 2018 and its Annual Report on Form 10-K for the year ended October 31, 2018. In August 2019, the Company engaged Marcum LLP as its independent registered public accounting firm. The Company expects these reports to be completed and filed during the first calendar quarter of 2020. Following completion and filing of these reports, the Company expects to promptly proceed to preparation and filing of its Quarterly and Annual Reports for the fiscal year ended October 31, 2019, with the objective of becoming current in its SEC reporting requirements as soon as possible.
Since November 2018, the Company has remained focused on research and development activities and sale and distribution of anti-aging and cellular therapy derived products.
In February 2019, the Company recommenced its efforts to once again operate a perinatal tissue bank processing laboratory in Miami, Florida for the purpose of performing research and development and the manufacturing and processing of anti-aging and cellular therapy derived products. This new laboratory facility became operational in May 2019 and during the same period, the Company began producing products that are now being sold and distributed to its customers.
In addition, the Company has created what it believes is a world class research, medical and scientific advisory team. We believe that our team is one of the most qualified and industry reputable teams assembled to adequately address the current and expected future medical and regulatory challenges facing the Company and overall industry and to provide leadership in the ongoing development of superior quality products for use in the health care industry.
The Company has actively taken steps to assure that it meets compliance with current and anticipated United States Food and Drug Administration (“FDA”) regulations expected to be enforced beginning in November 2020 requiring that the sale of products that fall under Section 351 of the Public Health Services Act pertaining to marketing traditional biologics and human cells, tissues and cellular and tissue based products (“HCT/Ps”) can only be sold pursuant to an approved biologics license application (“BLA”).
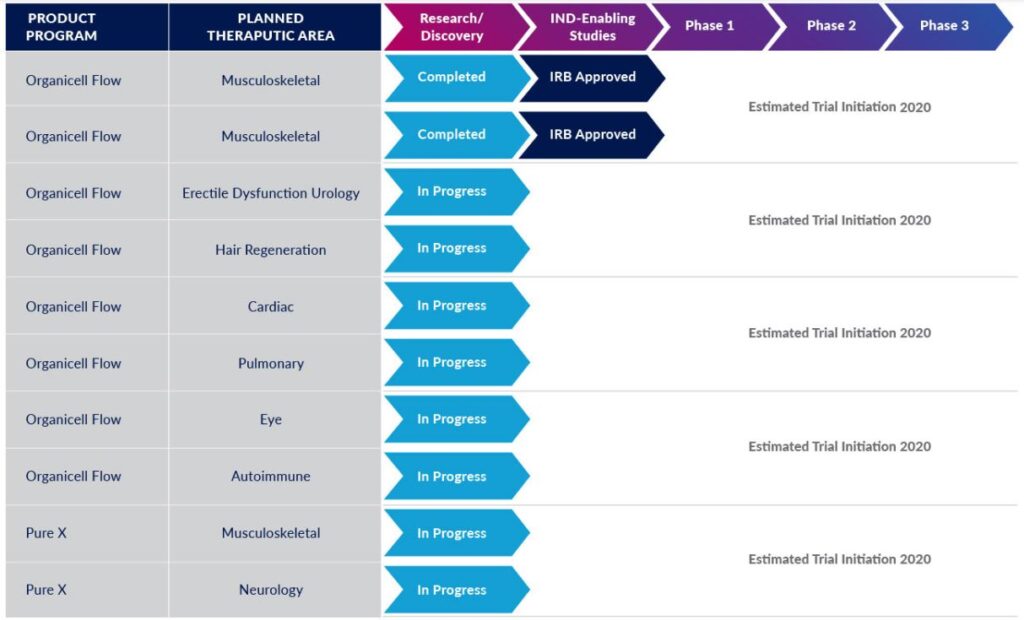
On July 14, 2019, the Company received Institutional Review Board (“IRB”) approval to proceed with two pilot studies in connection with the Company’s efforts to obtain Investigation New Drug (“IND”) approval from the FDA and commence clinical trials in connection with the use of the Company’s products and related treatment protocols for specific indications. The Company is aggressively pursuing efforts to obtain the aforementioned IND approvals and commence and complete those clinical studies as well as obtaining approval to commence additional studies for other specific indications it has identified that the use of its products will provide more favorable and desired health related benefits for patients seeking alternative treatment options than are currently available.
In an effort to increase sales and mitigate anticipated near future restrictions expected to be imposed by the FDA with respect to the use and distribution of Section 351 designated biologics, the Company is seeking to develop sales and distribution channels outside of the United States. In addition, the Company is focusing its efforts on developing other leading edge product offerings that would not fall within the FDA regulations for requiring a BLA license for U.S. manufacture and sale.
As a result of the Company’s expected future increase in processing requirements and to enable it to perform certain advanced research and development activities, the Company is currently in negotiations to relocate its laboratory facility during the second calendar quarter of 2020 to a larger “ISO 7” classified research and development and processing facility.
The Company has also been actively developing and expanding its sales, marketing and distribution network which it believes that based on the quality of the Company’s existing products, the Company’s commitment to regulatory compliance and superior research and development resources, the Company believes that it will be able to achieve desired growth during 2020.
The Company expects to provide periodic updates on operational and financial reporting developments as warranted.
For more information regarding the Company please visit our website at www.organicell.com.
About Organicell Regenerative Medicine, Inc.
Organicell is a leading, fully integrated Company focused in the field of regenerative medicine. Our world class research, technology, manufacturing and clinical development team is focused on creating new biologic medicines to revolutionize the field of regenerative medicine. We believe that our ground-breaking research in the field of nanotechnology, specifically exosome enrichments and other micro vesicles, is the next frontier of stem cell-based therapeutics. Organicell is committed to creating life changing and lifesaving therapies for patients.
Our mission is to transform regenerative medicine by continuing to combine exosome technology with other synergistic therapies and become the healthcare technology incubator for biologic medicine.
CAUTIONARY COMMENT REGARDING FORWARD-LOOKING STATEMENTS
The foregoing contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We intend for these forward-looking statements to be covered by the safe harbor provisions of the federal securities laws relating to forward-looking statements. This release contains forward-looking statements that reflect Organicell Regenerative Medicine Inc., and its subsidiaries, plans and expectations, financial situation, the ability to retain key personnel, product acceptance, the commercial success of any new products or technologies, success of clinical programs, ability to retain key customers, ability to expand sales and channels, and legislation or regulations affecting our operations and the ability to protect our patents and other intellectual property both domestically and internationally and other known and unknown risks and uncertainties. You are cautioned not to rely on these forward-looking statements. In this press release and related comments by Company management, words like “expect,” “anticipate,” “estimate,” “intend”, “believes” and similar expressions are used to identify forward-looking statements, representing management’s current judgment and expectations about possible future events.
Management believes these forward-looking statements and the judgments upon which they are based to be reasonable, but they are not guarantees of future performance and involve numerous known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, performance, achievements or financial position to be materially different from any expressed or implied by these forward-looking statements. Important factors that could cause actual results to differ materially from the forward-looking statements are set forth in our Form 10-K and other filings with the SEC. Other information can be obtained at www.organicell.com. The contents of the Company’s website are not incorporated by reference in this Press Release.
Specific information included in this press release may change over time and may or may not be accurate after the date of the release. Organicell has no intention and specifically disclaims any duty to update the information in this press releases.
CONTACT:
Organicell Regenerative Medicine Inc.
4045 Sheridan Ave.
Suite 239
Miami Beach, FL 33140
Website:http: www.organicell.com
Phone: (888) 963-7881
Email: info@organicell.com
*Cleaning up: (January 21, 2020) In August 2019, the Company engaged Marcum LLP as its independent registered public accounting firm. The Company expects these reports to be completed and filed during the first calendar quarter of 2020. Following completion and filing of these reports, the Company expects to promptly proceed to preparation and filing of its Quarterly and Annual Reports for the fiscal year ended October 31, 2019, with the objective of becoming current in its SEC reporting requirements as soon as possible.
Client see upcoming report for disclosure and disclaimer details.












