‘Bubble Boy’ Biotech Soars 250 Percent After Cure Announcement (Bloomberg).
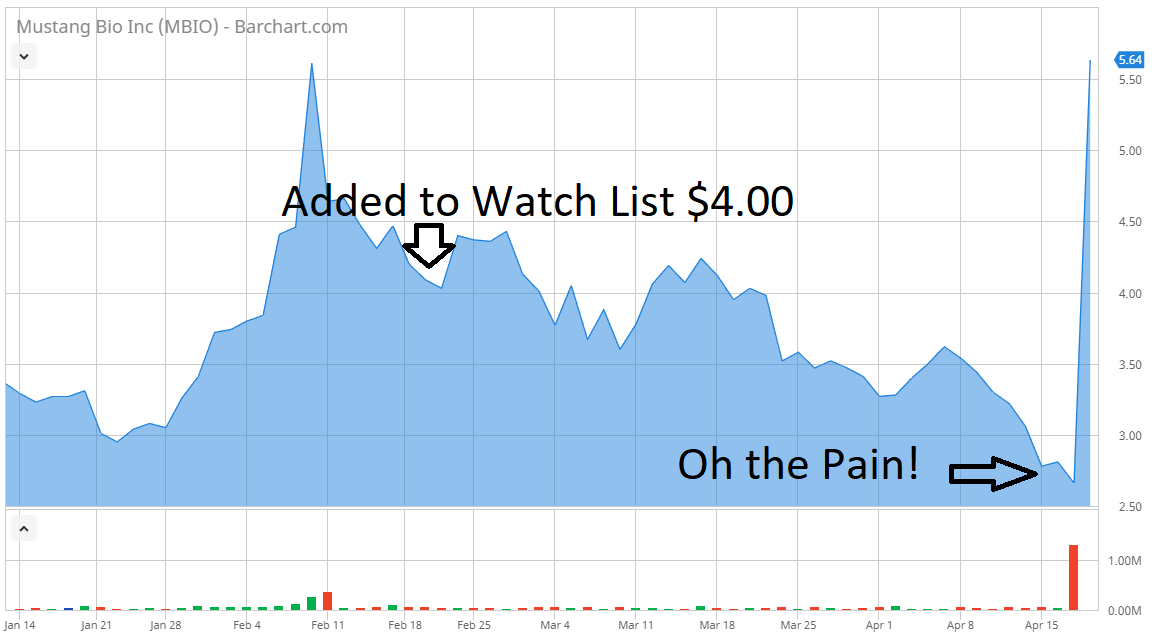
(Bloomberg) — Shares of the tiny biotechnology company Mustang Bio Inc. more than tripled Thursday, following a scientific breakthrough that cured eight infants suffering from the devastating “Bubble Boy” immune system disorder.
Mustang Bio, backed by health-care serial entrepreneur Lindsay Rosenwald, has licensed the treatment for the rare disease from St. Jude Children’s Research Hospital, where the treatments took place.
The shares were up 250 percent to $9.31 at 9:39 a.m. in New York, amid record volume, giving the company a market value of about $250 million.
Mustang Bio is an offshoot of Fortress Biotech Inc., which is run by Rosenwald and fellow entrepreneur Michael Weiss. Fortress, which specializes in investigating promising compounds and owns more than a third of Mustang Bio, also soared, by 57 percent.
“While I am a biotech entrepreneur, I am also a ‘risk manager,’ as we have many shots on goal for such small companies,” Rosenwald, who’s Fortress’s chief executive, said in an email. “The more shots on goal, theoretically the less risk there is.”
While he was confident about the potential for the gene therapy for the Bubble Boy disease, it has exceeded his expectations.
“I certainly believed this would work and work well, but I am surprised at this level of effectiveness with such a benign toxicity and side-effect profile,” he said.
Rosenwald owns about 13 percent of Fortress, and Weiss, who’s executive vice chairman, owns about 15 percent of the company, according to the latest data compiled by Bloomberg. Fortress, in turn, has a 38 percent stake in Mustang.
“Today we are in the sweet spot of medical research,” Rosenwald said. “There are more leads than there are people willing and able to try to move these sorts of things ahead.”
It’s very hard work, the executive said. “I love doing it.”
The one-time therapy is crafted for each individual patient by engineering stem cells from their bone marrow to express a healthy copy of a gene they didn’t get at birth. A study in the New England Journal of Medicine followed the first eight boys to get the therapy, tracking the development of a full immune system in each that allowed them to be released from the hospital and live normal lives.
Mustang Bio is focused mainly on developing novel immunotherapies for cancer and is still working to bring its first product to market. It plans to file for U.S. Food and Drug Administration approval for the gene therapy based mainly on the data in the publication, after it confirms the benefits in a handful of additional children treated with the therapy crafted in its Worcester, Massachusetts, manufacturing facility. The filing could occur by the end of 2021, said Chief Executive Officer Manny Litchman.
Once Mustang takes over the program from St. Jude at the end of the year, it will enroll every eligible child into its next trial, Litchman said. Regulators will continue to monitor their progress to ensure patients receive identical favorable results, he said.
Litchman said he doesn’t anticipate any problems with the Food and Drug Administration or other regulators making a decision based on the small number of patients, particularly since most of them don’t have a matched donor needed to get a bone marrow transplant that is considered the current standard of care. So far, 10 children with the condition known formally as X-linked severe combined immunodeficiency, or SCID, have been treated.
“The data are extraordinary for every single patient,” Litchman said. “It’s that compelling nature of the data, in particular for those who don’t have a matched sibling donor, that we believe will convince the FDA to do this. There is precedent in other rare diseases. We don’t think it’s crazy at all,” he said. “We need to be bold, we think it’s important to be bold for these kids who need this therapy.”
To contact the reporter on this story: Michelle Fay Cortez in Minneapolis at mcortez@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Cecile Daurat, Mark Schoifet
For more articles like this, please visit us at bloomberg.com
©2019 Bloomberg L.P.
Read full article here