RESIST THE RESISTANCE: A PRIMER ON ANTIBIOTICS & ANTIBIOTIC RESISTANCE (2013)
I was going to write this week about how DEET works, as a follow-up to last week’s post about “mosquito magnets.” But I decided to put that on hold after reading these four pieces that journalist and Superbug blogger Maryn McKenna, who writes about antibiotic resistance, published over the last couple of weeks on a group of bacteria called carbapenem-resistant Enterobacteriaceae, or CRE — what the director of the CDC is calling his “nightmare bacteria.”
It’s hard to believe that in the developed world in this day and age that you can walk into a hospital for surgery and come out dead, your body overwhelmed by lifeforms that you can’t see without a microscope. In the antibiotic age, that’s just not supposed to happen, right?
But it happens all the time. Antibiotic resistance is evolution in action. As McKenna notes, even Alexander Fleming, the father of the antibiotic age, feared that bacteria would come to resist his beloved penicillin. And because the ways we’ve misused and overused antibiotics over the mere 70 years they’ve been available to us, they’ve become almost useless in some cases.
Others have covered the issue of resistance much more eloquently than I. But what I thought I might do is, as with the glossary of fats, compile a list of what antibiotics are available to us today, what forms of resistance bacteria have evolved in response to them, and what we can and are doing to stop them.
How antibiotics work
Before we jump to the resistance, a note on how antibiotics work. Every antibiotic is either bacteriostatic — it prevents bacteria from growing and dividing — or bacteriocidal — it kills ’em dead. With a few exceptions that I note below, they do this by:
- Inhibiting protein synthesis (keeping bacteria from building proteins)
- Inhibiting cell wall formation (keeping them from building their cell walls)
- Inhibition of DNA replication and transcription (stopping them from reading or copying their DNA while dividing)
Let’s start with the families of antibiotics. I’ve included the names of one or two examples that you might have heard of from each family and how they work:
- Aminoglycosides (neomycin, amikacin). Inhibit protein synthesis.
- Beta-lactams. An antibiotic superfamily that includes the penicillins, cephalosporins, monobactams, and carbapenams. All of them contain what chemists call a beta-lactam ring made from three carbon atoms, a nitrogen atom, and an oxygen atom in their molecular structure. They generally block cell wall construction by keeping the bacteria from making a complex, interwoven layer of peptides and sugars called peptidoglycan.
- Carbapenems (imipenem). Block cell wall construction. They’re considered drugs of last-resort.
- Cephalosporins (cefotaxime, cefalexin). Block cell wall construction.
- Glycopeptides (vancomycin). Same as cephalospoins.
- Lincosamides (clindamycin). Stop protein synthesis.
- Lipopeptides (daptomycin). This one is unique. It pokes holes in the bacterial cell membrane, allowing ions inside the cell to leak out. That messes with the cell’s electrochemistry and keeps it from producing protein or synthesizing new DNA or RNA.
- Macrolides (erythromycin, azithromycin). Stop protein synthesis.
- Monobactams (aztreonam). Block cell wall construction.
- Nitrofurans (furazolidone). May cause segments of bacterial DNA to stick together, or crosslink.
- Oxazolidonones (linezolid). Stop protein synthesis.
- Penicillins (penicillin methicillin, amoxicillin). The granddaddy of the antibiotics. Block cell wall production.
- Polypeptides (bacitracin, colistin, polymyxin B). Either block cell wall production (bacitracin) or interfere with the bacterial cell membrane. Doctors are increasingly turning to colistin, an older antibiotic, to treat infections by bacteria resistant to multiple other antibiotics, despite its severe side effects.
- Quinolones (ciprofloxacin). Prevent DNA replication and transcription.
- Sulfonamides (trimethoprim-sulfamethoxazole). These drugs keep bacteria from synthesizing folate (one of the B vitamins), which keeps them from producing the nucleic acid building blocks of DNA and RNA.
- Tetracyclines (tetracycline, doxycycline). Stop protein synthesis.
Read the full great article here.
What’s amazing is that many antibiotics are derived from microbes themselves. Penicillin was famously discovered in a penicillium mold that had contaminated one of Alexander Fleming’s petri dishes; vancomycin was first isolated from soil bacteria. They’re like the chemical weapons of the microbial world, giving the microbes that produce them a competitive edge or means of defense against others.
How bacteria resist antibiotics
Now let’s dive into the resistance. Generally speaking, resistant bacteria fight off antibiotics in one of five ways:
- Reduce cell wall permeability (tighten up the cell wall so the drugs can’t get in)
- Enzymatic inactivation (use enzymes to turn the drug off or chew it up)
- Change the target of the antibiotic (mutate the gene for whatever protein it is that the antibiotic goes after)
- Efflux pump (use of a protein that actually pumps the drug out of the bacteria before it can do much harm)
- Bypass the inhibition (mutate such that the bacteria can get more nutrients and other necessary products from their environment, rather than producing their own)
All of these mechanisms stem from genes, either changes in existing ones or evolution of new ones. And bacteria have this nasty habit (nasty for us, great for them) of passing resistance genes directly between each other, even between different species. This is why the same resistance mechanism is often shared among multiple species of bacteria.
The resistance is full of acronyms and weird words; here are the ones you’re most likely to see and hear, the ones that have the CDC and their equivalents around the world scared. Mind you, this nowhere close to a full list of the kinds of antibiotic resistance out there — resistance has been found to nearly every antibiotic at some level — but just the names and acronyms of the kinds that are the most worrisome at the moment:
- Beta-lactamase. A class of enzymes that cut up antibiotics with beta-lactam rings. A lot of different bacterial species have either evolved their own beta-lactamases or obtained them from other species.
- CRE (carbapenem-resistant Enterobacteriaceae). As I mentioned above, these are the CDC’s nightmare bacteria. (CDC administrators aren’t typically given to hyperbole. If they’re using language like that, it has to be bad.) The Enterobacteriaceae — also called enterobacteria (because many of them are found in the gut) — are a large family that includes bugs with melodious names like Escherichia coli, Klebsiella pneumoniae, and Salmonella enterica. So far CREs have only been found in patients who are already sick and in a hospital setting like an intensive care unit or long-term care ward. Some of the CREs are resistant to pretty much everything in our antibiotic arsenal.
- KPC (Klebsiella pneumoniae carbapenemase). An enzyme that breaks down carbapenem-family antibiotics, one of the two mechanisms by which CREs resist the carbapenems.
- MDR-TB (mulitdrug-resistant tuberculosis). Refers to strains of the tuberculosis bacterium, Mycobacterium tuberculosis, that are resistant to at least the two most potent anti-TB drugs, isoniazid and rifampin. Drug-resistant TB arose largely because many TB patients historically did not complete the long regimen of drug treatment for the disease.
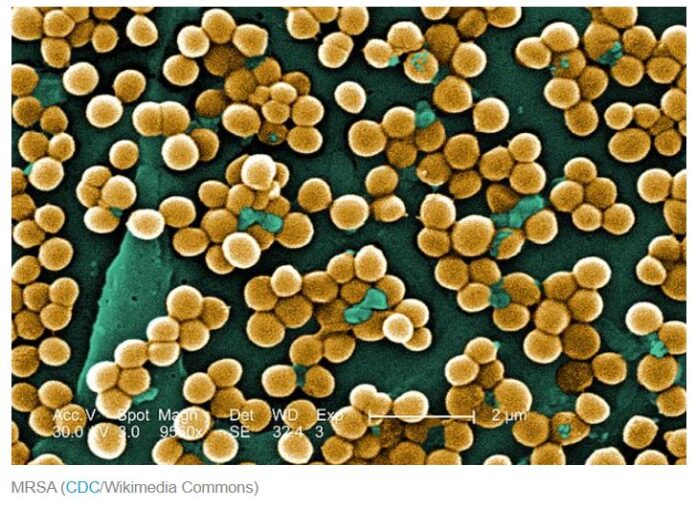
- MRSA (methicillin-resistant Staphylococcus aureus). Strains of staph bacteria that are resistant to the beta-lactams. In the general population they most often show up as skin infections, while in hospitals and other healthcare settings they can appear throughout the body. People can be infected or colonized with MRSA; if you’re colonized you carry it around with you but show no symptoms.
- NDM-1 (New Delhi metallo-beta-lactamase-1). The other known carbapenem-destroying enzyme. It was first found in 2009 in a patient in Sweden who was infected with drug-resistant E. coli and Klebsiella pneumoniae at the same time.
- VISA (vancomycin intermediate Staphylococcus aureus) and VRSA (vancomycin-resistant Staphylococcus aureus). Two types of staph bacteria that resist the antibiotic vancomycin, though they are usually sensitive to other antibiotics. The difference between the two lies in the amount of vancomycin it takes to kill them in laboratory tests.
- VRE (vancomycin-resistant enterococci). Bacteria normally found in the gut that have become resistant to vancomycin and usually other drugs as well. As with MRSA, people can be colonized or infected. Usually found in healthcare settings like hospitals.
- XDR-TB (extensively drug-resistant TB). Tuberculosis that is resistant to nearly every drug available for TB, including isonizaid and rifampin. Because doctors have to turn to drugs that aren’t as effective against TB in order to treat it, patients fare much worse. It’s a rare sub-form of MDR-TB, but is growing more common.