Down 20% in response to news in an 8K that the Biomedical Advanced Research and Development Authority BARDA (also once known as the richest kid on the block with a $5 billion endowment) has canceled their plans to fund one of GeoVax’s Covid Vaccine clinical trials.
As we mentioned last month, despite headwinds affecting Moderna (MRNA), Novavax (NVAX), Sarepta (SRPT) and seven others, we just felt GeoVax (GOVX) would be able to sidestep the JFK related issues, beacuse..well..BARDA promised. It’s not like ‘not getting’ an anticipated contract. This was signed and sealed. Oh well – no guarantees in the stock market and ecspecially in the biotech sector.
Vaccine Biotechs Hammered After FDA Official Resigns, Citing RFK Jr.’s ‘Misinformation And Lies.’
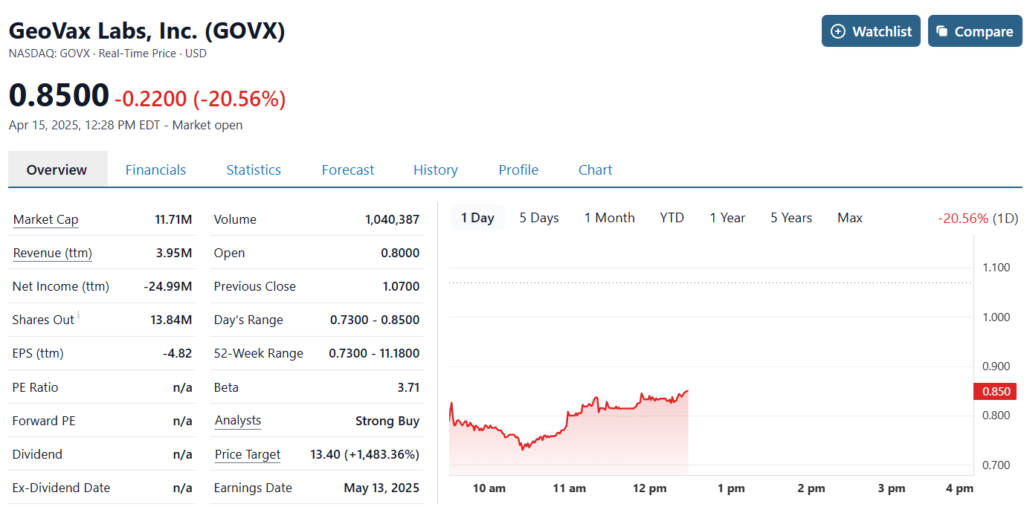
They worded the cancellation as “BARDA decided to terminate the contract for convenience.” Wait, what. But you promised! Convenience? You rat bastards lol.
Barda, who conducted an indepth study on the vaccine (initially for the immunocompromised), was so impressed with what they saw, and its potential to save lives – they told GeoVax it would fund their clinical trials! That’s called non-dilutive financing at its best.
Like a rich uncle at an expensive French restaurant, who picks up the check for a party of ten, saying “Put your money away, Uncle Bart is getting this. Your money is no good here.”
Except the dinner check was $400 million. Talk about generous! “We love you Uncle Bart, another bottle of Champagne waiter – please.”
But then Uncle Bart does a rug pull at the last minute, when the check arrives. You would have never drank those $300 bottles of Champagne! That rat bastard lol.
So our condolences to the project, the folks and and hard working researchers at Barda, who just wanted to make the world a safer place to live.
Fortunately, it is not game over to GeoVax. Managment is brilliant (which is how the got the grant in the first place over 100’s of others), and more importantly ‘scrappy.‘ The data to date, has all been ‘proceed’ and ‘keep going.’ Data to date, has said it’s both safe and effective. They just need a broader patient base to undergoe treatment and collect data. If anyone can get this to a priorty review for approval, we beleive they can.
We will suspend coverage which is hard, because GeoVax has, as we have said many times, our favorite start-up. Suspend doesn’t mean no longer following them. It just means we have no idea of their upcoming plans, and that we’ll resume coverage once things settle down. By ‘things’ we mean how investors will react to things. We have no clue.
The Covid vaccine was never our favorite shot on goal as the panic fizzled out. Our favorites in order:
- Gedeptin, combined with a checkpoint inhibitor.
- The Monkeypox production line vs Bavarian Nordic (BVRNY)
- The Covid vaccine for chronic lymphocytic leukemia (CLL) with patients being tested at the world renowed City of Hope.
GeoVax Reports 2024 Year-End Financial Results and Provides Business Update
In our wildest of dreams, with a little more proof of safety and efficacy we believe GeoVax can be acquired or partnered with, once again paving the road with gold. It’s JUST not going to be as fast or simple (funding issues) as it was last week.
On the other hand, this event may encourage a billion dollar pharaceutical to strike now. While the iron is hot. Maybe at tomorrow’s International Vaccine Conference.
While we are getting a little old for this (67 next week), micro-caps under distress will remain our sweet-spot now and forever.
UNRELATED: Adding Allarity (ALLR) $0.70, to Watch List.
DISCLAIMER
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.
Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law. Client, see report for disclosure and disclaimer details.












