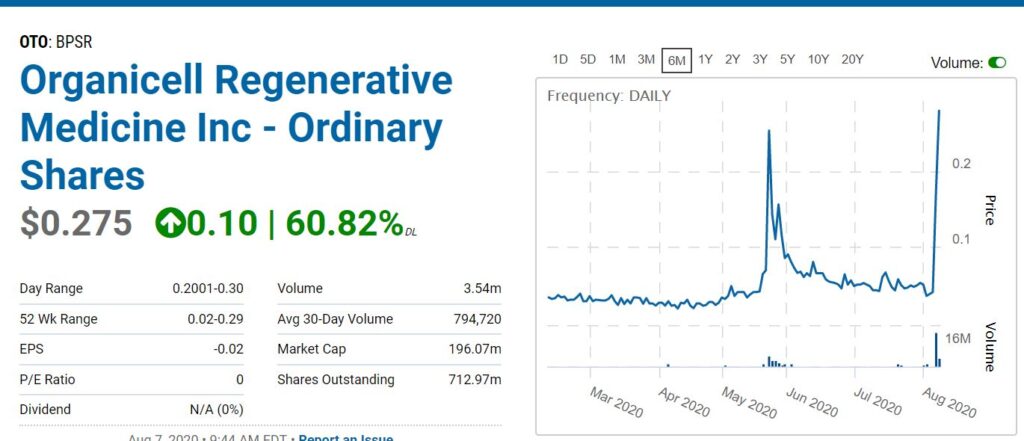
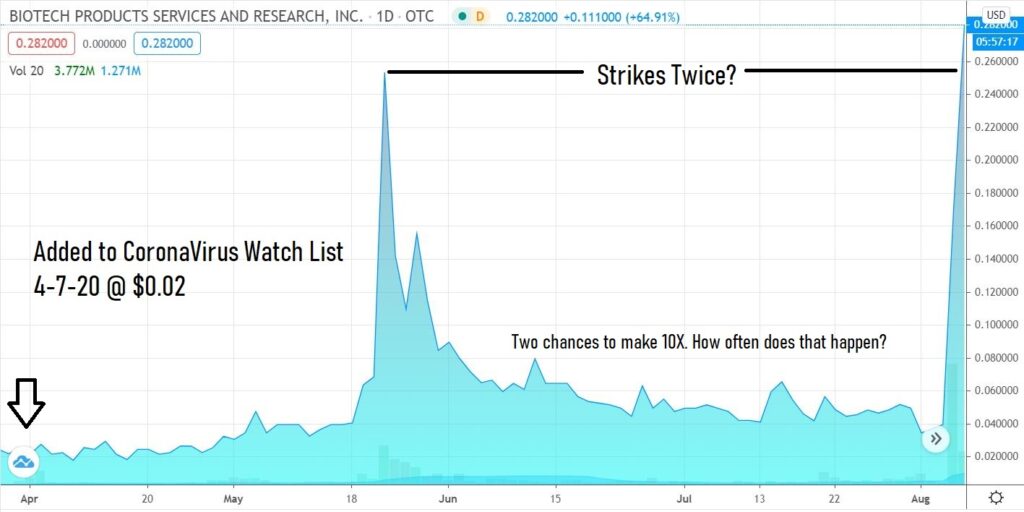
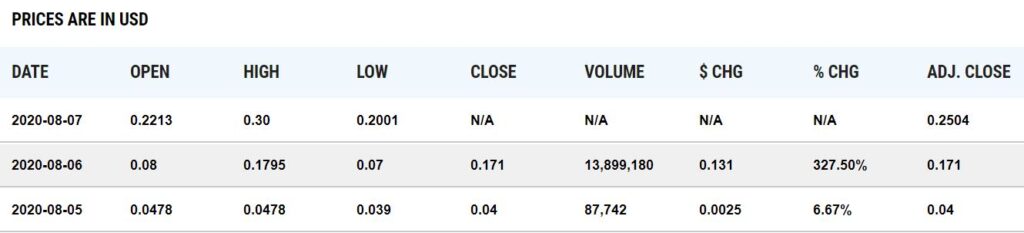
Up on news, how does a stock go from 87,000 shares traded to 13 million traded the next day? Corona Power!
Another day another 10-bagger in Corona Mania world. You can’t make this up.
We’ll have the 2020 CoronaVirus Investor Guide released Monday (or Tuesday). It’ll be a bevy, a treasure trove of ideas – covering vaccines, anti-virals, sanitizers, testing kits, telehealth, and even the camping and RV life!.
We’ve already found four (including Organicell) whch ran up 10-fold, can we find more?
Only time will tell and only for subscribers – Subscribe below – and tell two friends.
Wait, what? The RV Life? Look at the chart on Marcus Leonis Camping World (CWH).
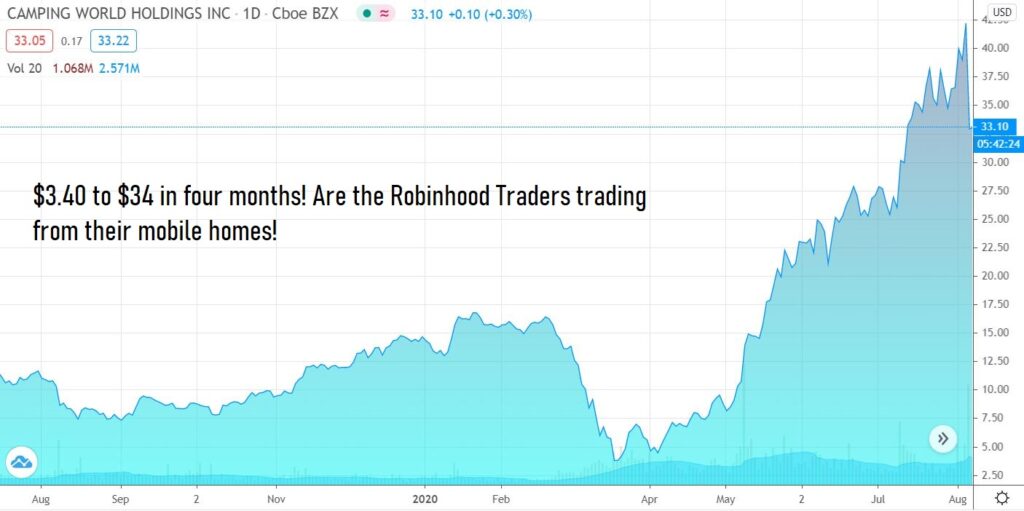
“As the single largest shareholder with beneficial ownership of over 36 million shares and a steward for our nearly 11,000 associates, I feel strongly that our goals and focus as shareholders are aligned.”
FDA Approves Two Emergency INDs for COVID-19 Outpatient Therapy
First Reported eIND Issued for Outpatient COVID-19 Therapy
MIAMI, Aug. 06, 2020 (GLOBE NEWSWIRE) — Organicell Regenerative Medicine, Inc. (OTCBB: BPSR), a clinical-stage biopharmaceutical company dedicated to the development of regenerative therapies, announced today that the U.S. Food and Drug Administration (FDA) approved two outpatient Emergency Investigational New Drug Applications (eINDs) for treating mild to moderate respiratory distress due to COVID-19.
Organicell’s first patient approved for the eIND experienced moderate chest discomfort accentuated when taking deep breaths, persistent non-productive cough and fatigue upon exertion. After receiving three doses of Organicell Flow, the patient has shown a reduction in cough and ability to breathe without pain. The second outpatient previously spent seven weeks in the hospital, was admitted to the ICU twice, received Bi-Level Positive Airway Pressure (BiPAP) treatment and continued to experienced shortness of breath after hospital discharge. Now, being treated as an outpatient, he was administered the first dose of Organicell Flow on August 4th, 2020, and to date, has reported improvements in all his symptoms.
In May, Organicell previously announced it had successfully treated two critically ill COVID-19 inpatients at Landmark Hospital in Athens, Georgia. Since then, a third approved eIND inpatient with COVID-19 related respiratory impairment and acute kidney injury, was treated by Organicell, and the patient had a complete recovery of lung and renal function and was discharged 26 days after initiation of treatment.
Follow CoronaMania stocks on our SuperBug News section of the Biotech Stock Review.
Sign Up for our upcoming 2020 Corona Virus Investors Guide.
2020 Biotech Portfolio Soars 55%, We’re Amazed. Next Up: The Coming Gold Rush.

Many clients involved, see reports for disclosure and disclaimer details.












