Innovative Innovus now has Three Shots on Goal with non-prescription drugs which have come off patent including a new competitor to Prilosec ® .
This gets more interesting by the day. Innovus seeks out blockbuster drugs which are off patent AND are, or expected to, change from prescription (or Rx) to OTC. When this happens (no longer being Rx) prices drop, the product becomes more widely available (you can just buy off the shelf) and marketing wars commence. In this war, Innovus in three short years has proven to be a Ninja like warrior in the battle for consumer’s eyes and wallets. The best thing is that there is no ‘new-drug’ experimentation and risk. We know these drugs are safe. Safe enough to be bought without a prescription. We also know these drugs are in high demand. And now anyone can buy them opening sales to millions more.
It’s a war of the best pricing and marketing. And literally, billions are at stake. The Innovus new names and packaging are cleverly similar – so consumers don’t have to be a rocket scientist to think “Hmm, this is similar and cheaper, I think I’ll try this instead.”

The Company markets directly, (a) commercial partners to primary care physicians, urologists, gynecologists and therapists, and (b) directly to consumers through dozens of their own online channels, retailers and wholesalers.
THREE BLOCKBUSTER DRUGS INNOVUS IS TARGETING.
Fluticasone™ vs. Flonase® .
Regoxidein™ vs Rogaine® .
OmepraCare™ vs Prilosec®
Together those three products alone are starring an addressable into the billions. And Innovus wants its share of the pie. Even a small slice is huge. And ironically, the tens of millions that the competing drug companies spend advertising benefits Innovus because it brings consumers to the ‘category,’ not just the drug advertised!
We advise readers to note, the Company recently underwent a 105:1 reverse split in seeking to graduate to a more senior exchange now that they have left development stage (over $20 million in sales) and more in line with their status as a “bonafide growth company.” While this may present some problems as older shareholders give up, new investors can try (with a good dose of patience) to build a stake while liquidity is temporarily out of whack – in a company which we believe has a tremendous future. Therein our opinion lies the opportunity. Once trading gets back to normal, this could run strong – there is not a lot of stock available after the reverse.
Secondly, the Rogaine® and Prilosec® competing products aren’t expected to be released until later this year and if there are early reports of success similar to the success they’ve been having with Fluticare, it could rapidly propel the share price higher and there is not a lot of stock available.
RELATED: Innovus Pharmaceuticals Reports Preliminary Q1 2019 Sales Revenue of Approximately $5.4 million
Innovus Pharma Expands its OTC Drug Pipeline with the Addition of OmepraCare™ (a Competitor of Prilosec® OTC)
Innovus Pharmaceuticals, Inc. (“Innovus Pharma” or the “Company”) (OTCQB Venture Market: INNV), an emerging commercial-stage pharmaceutical company that delivers safe, innovative and effective over-the-counter medicine and consumer care products to improve men’s and women’s health and respiratory diseases, today announced that it has entered into a supply relationship with a third party to supply Innovus Pharma with omeprazole 20mg tablets and omeprazole 20mg 24 hour delayed-release capsules indicated for the treatment of frequent heartburn under the approved abbreviated new drug application (“ANDA”) No. 207891 from the U.S. Food and Drug Administration (“FDA”). Innovus Pharma will launch the drug under its own trademark OmepraCare™ 20mg tablets and OmepraCare™ DR 20mg 24 hour delayed-release capsules.
“We continue to execute on our plan to launch new OTC drugs in niche therapeutic areas where there is a need for national brands to leverage our success with FlutiCare® and other drugs and devices we have in the United States. OmepraCare DR™ is the third ANDA OTC drug that we intend to commercialize in the U.S. this year,” said Dr. Bassam Damaj, Innovus Pharma’s President and Chief Executive Officer. “We believe that the market opportunity for us with this product is fairly large given the success of the product Prilosec® OTC and the relatively large size of the market. Our goal is to launch OmepraCare™ in the US in the second half of this year,” added Dr. Damaj.
“In addition, it is our current intention to add up to 10 additional ANDA OTC products to our US drug pipeline,” he continued.
About OmepraCare
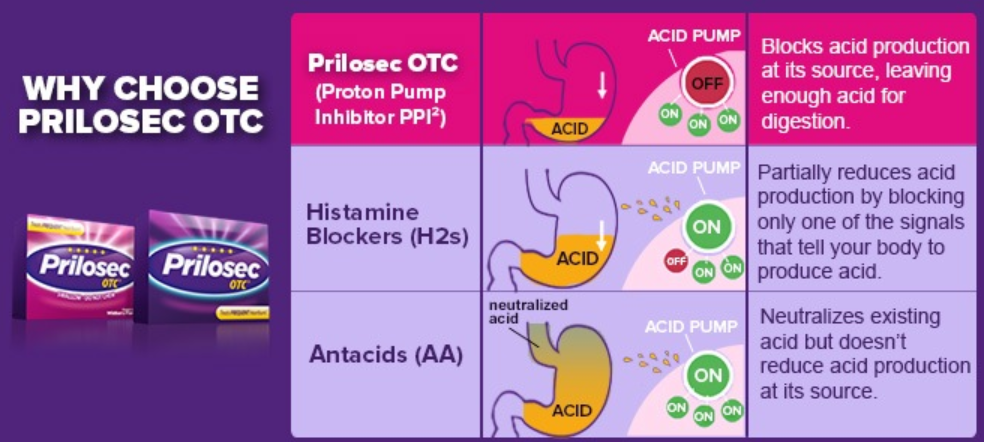
OmepraCare™ which is an OTC branded version of omeprazole and a competitor of Prilosec® OTC, is a proton pump inhibitor to reduce stomach acidity. It contains the #1 Doctor† recommended active drug molecule for treatment of frequent heartburn for 10 straight years.
OmepraCare™ is different from many other heartburn treatments. It works by blocking the acid that causes heartburn and lasts 24 hours with one pill each morning. It will be available at the 20mg dose tablets and 24 hour delayed release capsules in 42 counts.
It is on the World Health Organization’s List of Essential Drugs which lists the most effective and safe medicines needed in a health system. It was the seventh most prescribed medication in the United States, with more than 70 million prescriptions in 2016. OmepraCare™ is comparable to Prilosec® OTC. Coherent Market Insights has estimated that in 2017, the global omeprazole market size was valued at approximately $2.67 billion and is expected to have an increasing CAGR of 5.2% over the 2018 to 2026 time period.
About Innovus Pharmaceuticals, Inc.
Headquartered in San Diego, Innovus Pharma is an emerging OTC consumer goods and specialty pharmaceutical company engaged in the commercialization, licensing and development of safe and effective non-prescription medicine and consumer care products to improve men’s and women’s health and vitality and respiratory diseases. Innovus Pharma delivers innovative and uniquely presented and packaged health solutions through its (a) OTC medicines and consumer and health products, which we market directly, (b) commercial partners to primary care physicians, urologists, gynecologists and therapists, and (c) directly to consumers through our on-line channels, retailers and wholesalers. The Company is dedicated to being a leader in developing and marketing new OTC and branded Abbreviated New Drug Application (“ANDA”) products. The Company is actively pursuing opportunities where existing prescription drugs have recently, or are expected to, change from prescription (or Rx) to OTC.
For more information, go to www.innovuspharma.com; www.zestra.com; www.ejectdelay.com; www.myvesele.com; www.urivarx.com; www.sensumplus.com; www.myandroferti.com; www.beyondhumantestosterone.com;www.getbeyondhuman.com; www.trybeyondhuman.com; www.recalmax.com; www.prostagorx.com;www.xyralid.com; www.fluticare.com; www.allervarx.com; www.apeaz.com; www.regoxidine.com and omepracaredr.com.
Innovus Pharma’s Forward-Looking Safe Harbor:
Statements under the Private Securities Litigation Reform Act, as amended: with the exception of the historical information contained in this release, the matters described herein contain forward-looking statements that involve risks and uncertainties that may individually or mutually impact the matters herein described for a variety of reasons that are outside the control of the Company, including, but not limited to, projected revenues from OmepraCare™, estimated market for its products, and statements about achieving its other development, growth, commercialization, financial and staffing objectives. Readers are cautioned not to place undue reliance on these forward-looking statements as actual results could differ materially from the forward-looking statements contained herein. Readers are urged to read the risk factors set forth in the Company’s most recent filing on Form S-1, annual report on Form 10-K, subsequent quarterly reports filed on Form 10-Q and other filings made with the SEC. Copies of these reports are available from the SEC’s website or without charge from the Company.
*Prilosec® is a trademark owned by Proctor and Gamble and Astra Zeneca. †https://www.prilosecotc.com/en-us/check-the-facts
Client, please see report disclaimer for details.












