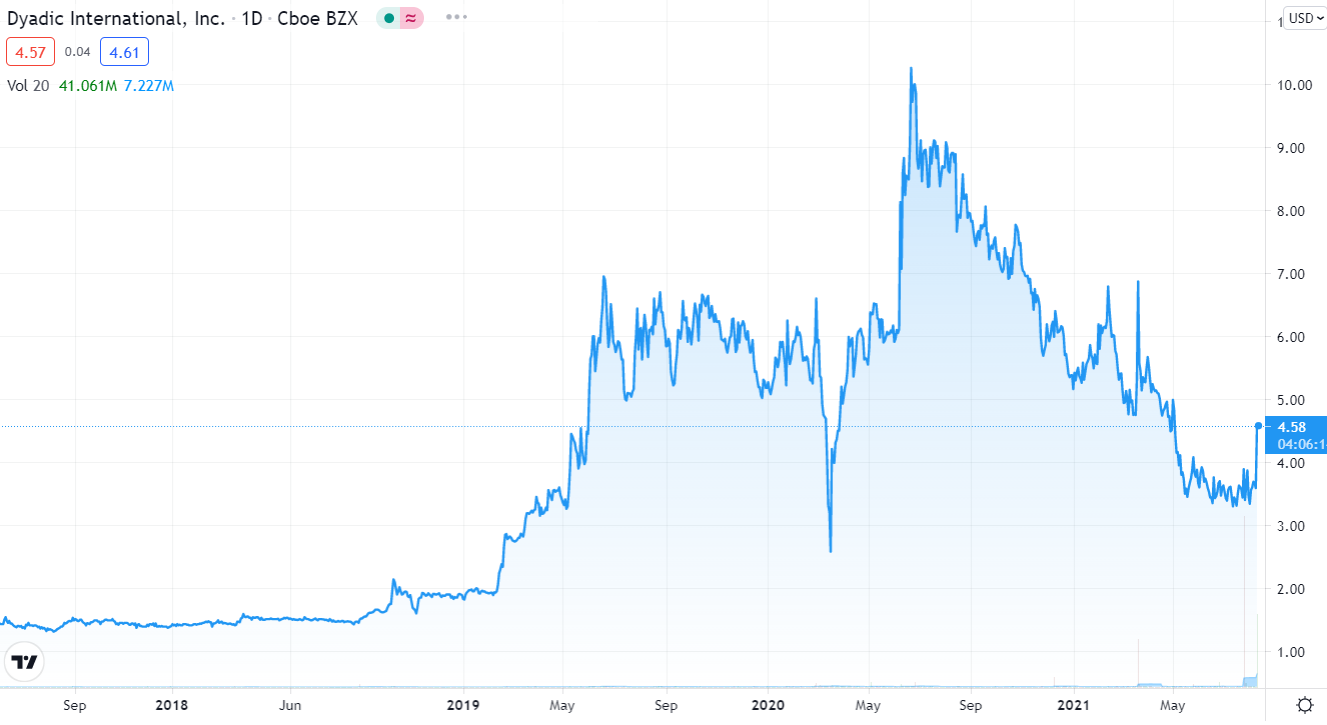
Share Price up 27% ($4.63 up $1.04) as Wall Street Sorts out the Terms of the Deal.
We’ve had a Long History With Dyadic Dating Back to August of 2017
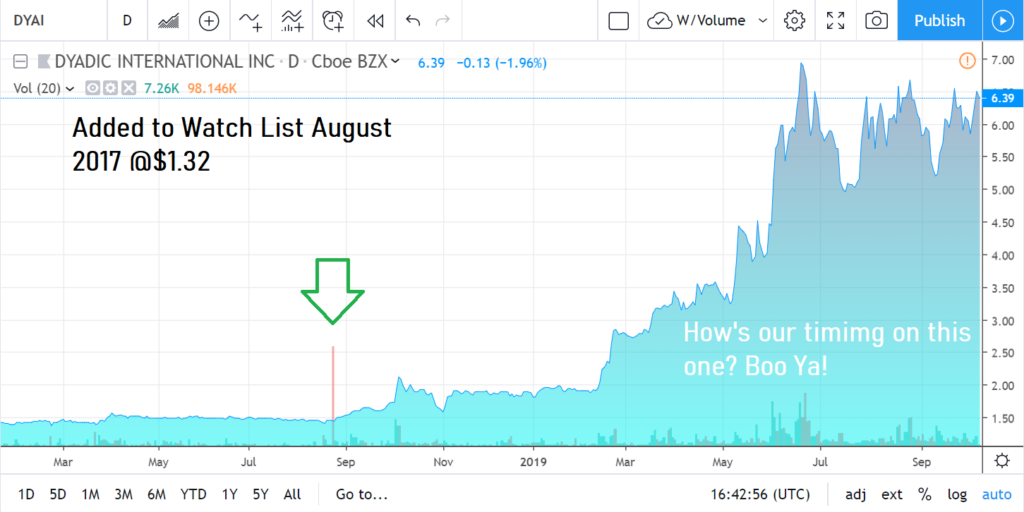
At its peak in June of 2020 we were up 667% when it traded as high as $10.26. But it has been rough sledding throughout 2021 as the chart below shows.
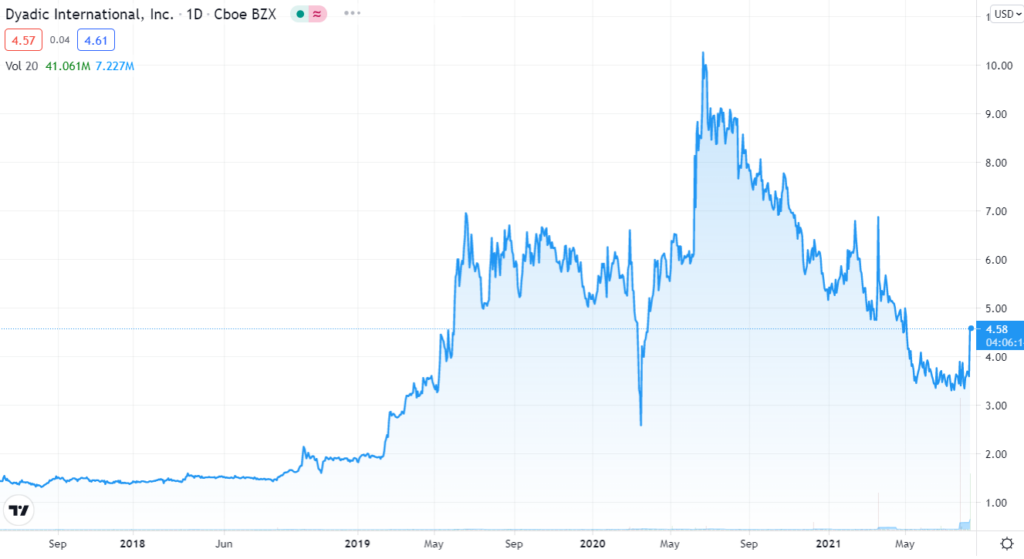
Despite the volatility, what we like most about Dyadic is that there lead product C1-cell protein production platform isn’t and end use product, but rather a technology which can be used by other pharmaceutical companies. Quite simply, their technology can other companies accelerate development, lower production costs and improve access to biologic vaccines and drugs at flexible commercial scales.
The beauty of this (if you’re patient) is that new licensing or partnership deals can come at the drop of a hat – and since they aren’t ‘expected’ or baked into the stock price, the shares can run like the dickens.
Sorrento and Dyadic Announce Binding Term Sheet to License Dyadic’s Lead COVID-19 Vaccine Candidate “DYAI-100” and C1 Technology for Protein-Based Coronavirus Vaccines and Therapeutics
“The license agreement, when executed, contemplates an up-front payment of $10 million in cash and stock, up to $4 million in reimbursements for preclinical and clinical development costs incurred by Dyadic for the development and advancement of our proprietary DYAI-100 vaccine, up to $33 million in milestone payments and ongoing royalties..”
Past client see report for disclosure and disclaimer details.
This press release and any statements made for and during any presentation or meeting contain forward-looking statements related to Sorrento Therapeutics, Inc., under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements regarding the C1-cell protein production platform and DYAI-100; the expected collaboration between Sorrento and Dyadic, including but not limited to, the development and commercialization of COVID-19 vaccines, including DYAI-100 and Sorrento’s MultiValent COVID-19 vaccine, and therapeutic antibodies, protein therapeutics and diagnostics for coronaviruses; the properties of DYAI-100, including its induction of high-titer neutralization activity against SARS-CoV-2 and its variants of concern; the capability to manufacture protein-based vaccines in large quantities; Sorrento’s resources and expertise in developing and commercializing vaccines, therapeutics and diagnostics; the potential for such protein-based vaccines to be stored and transported at room temperature; the expectation that such vaccines would be effective against SARS-CoV-2 and its variants of concern; the plan to utilize the C-1 cell protein production platform in connection with other existing and new coronavirus programs; and the expected entry into a definitive license agreement between the parties and the terms thereof. Risks and uncertainties that could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements, include, but are not limited to: risks related to Sorrento’s technologies and prospects, including, but not limited to risks related to seeking regulatory approval for DYAI-100 and other vaccine and therapeutic candidates produced by the C1-cell protein production platform; clinical development risks, including risks in the progress, timing, cost, and results of clinical trials and product development programs; risk of difficulties or delays in obtaining regulatory approvals; risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval; risks that prior test, study and trial results may not be replicated in future studies and trials; risks of manufacturing and supplying drug product; risks related to leveraging the expertise of its employees, subsidiaries, affiliates and partners to assist Sorrento in the execution of its therapeutic antibody product candidate strategies; risks related to the global impact of COVID-19; and other risks that are described in Sorrento’s most recent periodic reports filed with the Securities and Exchange Commission, including Sorrento’s Annual Report on Form 10-K for the year ended December 31, 2020, and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release and we undertake no obligation to update any forward-looking statement in this press release except as required by law.
$DYAI, #DYAI