CRANFORD, N.J., May 13, 2019 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius”) (Nasdaq: CTXR), a specialty pharmaceutical company focused on adjunctive cancer care and critical care drug products, provided a report today on the in vitro efficacy of Mino-Lok against various strains of Candida auris.
CDC: C. AURIS, A GLOBAL THREAT
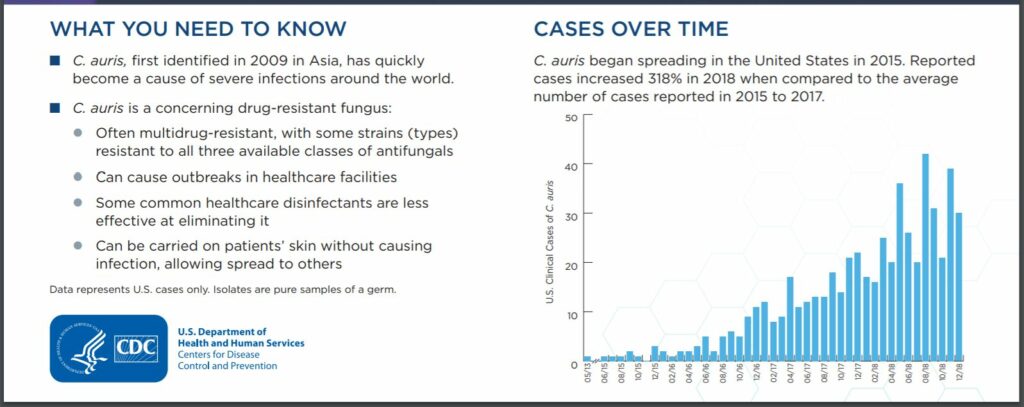
Candida auris (“C. auris“) is a fungus that causes serious infections. C. auris can cause bloodstream infections and even death, particularly in hospital and nursing home patients with serious medical problems. More than 1 in 3 patients with invasive C. auris infection die. It’s often resistant to most antifungal agents commonly used to treat Candida infections. Although C. auris was just discovered in 2009, it has spread quickly and caused infections in more than a dozen countries. Patients who have been hospitalized in a healthcare facility a long time, have a central venous catheter, or other lines or tubes entering their body, or have previously received antibiotics or antifungal medications, appear to be at highest risk of infection with this yeast.
Mr. Myron Holubiak, CEO of Citius, said, “We are very enthused Mino-Lok has shown strong activity against a significant number of C. auris strains and that complete eradication occurred within an hour of exposure. Fortunately, our patients have not encountered any C. auris CLABSIs to date in our clinical trials.
While these data for Mino-Lok are highly encouraging as they relate to Candida, in vitro data do not necessarily correlate with clinical results; and, we recognize more work needs to be done. The company is currently studying Mino-Lok in a phase 3 trial in over 40 participating institutions, all located in the U.S.”
RELATED: CDC’S 2019 BIGGEST THREAT REPORT
Blood stream infections due to Candida auris are an emerging public health concern due to high prevalence of antifungal resistance and significant rates of patient mortality. C. auris is typically highly resistant; and, several strains have been identified with elevated MICs to all major classes of antifungals. Previously Citius reported that Mino-Lok was highly effective in clinical trials that evaluated salvage of catheters in patients with highly virulent bacterial infections. In this laboratory study, Mino-Lok was evaluated in its ability to eradicate C. auris in mature biofilms in vitro within an hour of exposure*.
* Data on file.
Mino-Lok®
Mino-Lok is an antibiotic lock solution used to treat patients with CLABSIs/CRBSIs. CLABSIs/CRBSIs are very serious, especially in cancer patients receiving therapy through central venous catheters (CVCs), and in hemodialysis patients for whom venous access presents a challenge. There are currently no approved therapies to salvage infected central venous catheters (CVCs).
About Citius Pharmaceuticals, Inc.
Citius is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products, with a focus on anti-infectives, cancer care and unique prescription products that use innovative, patented or proprietary formulations of previously approved active pharmaceutical ingredients. We seek to achieve leading market positions by providing therapeutic products that address unmet medical needs. By using previously approved drugs with substantial safety and efficacy data, we seek to reduce the risks associated with pharmaceutical product development and regulatory requirements. Citius develops products that have intellectual property protection and competitive advantages to existing therapeutic approaches. For more information, please visit www.citiuspharma.com.
Safe Harbor
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements are made based on our expectations and beliefs concerning future events impacting Citius. You can identify these statements by the fact that they use words such as “will,” “anticipate,” “estimate,” “expect,” “should,” and “may” and other words and terms of similar meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price.
Factors that could cause actual results to differ materially from those currently anticipated are risks relating to the results of research and development activities, including that preclinical results may not be replicated in clinical trials; uncertainties relating to preclinical and clinical testing; patent and intellectual property matters; risks associated with conducting our phase 3 trial for Mino-Lok, including completing patient enrollment and opening study sites; the estimated markets for our product candidates and the acceptance thereof by any market; risks related to our growth strategy; the early stage of products under development; our ability to obtain, perform under, and maintain financing and strategic agreements and relationships; our ability to identify, acquire, close, and integrate product candidates and companies successfully and on a timely basis; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; our need for substantial additional funds; government regulation; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
Contact:
Andrew Scott
Vice President, Corporate Development
(o) 908-967-6676
[email protected]
View original content:http://www.prnewswire.com/news-releases/citius-pharmaceuticals-mino-lok-highly-efficacious-in-rapidly-eradicating-candida-auris-300848639.html
SOURCE Citius Pharmaceuticals, Inc.
#CTXR, #Citius, #Candida Auris













