2021 and 2020 Milestones In Plain Sight.
Locked and Loaded for most of 2020 – many major milestone events lie around the corner for Citius Pharma (CTXR). In the biotech sector, ‘around the corner’ can mean 5 years even more – and tens if not $100’s of millions in trial work. This is not the case with Citius. What we are talking about here, is significant news in the next 12 months.
IMMUNOMEDICS (IMMU) 35 YEARS TO ‘AROUND THE CORNER‘
ACQUIRED AT $86
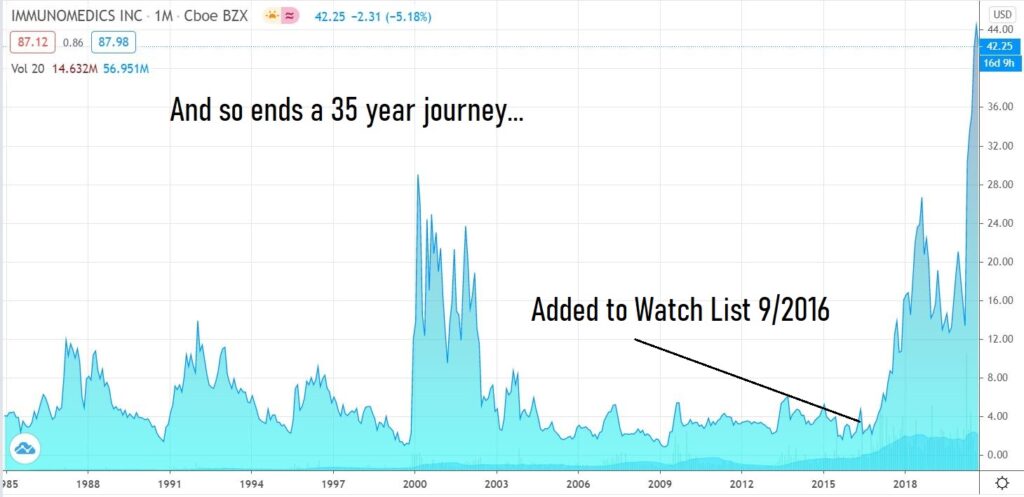
Most of 2020 has been a traders paradise in Citius, with numerous 30% intra-month gains on news that they made yet another step to their goal of getting to the potentially life-saving Mino-Lok anti-bacterial product to market.
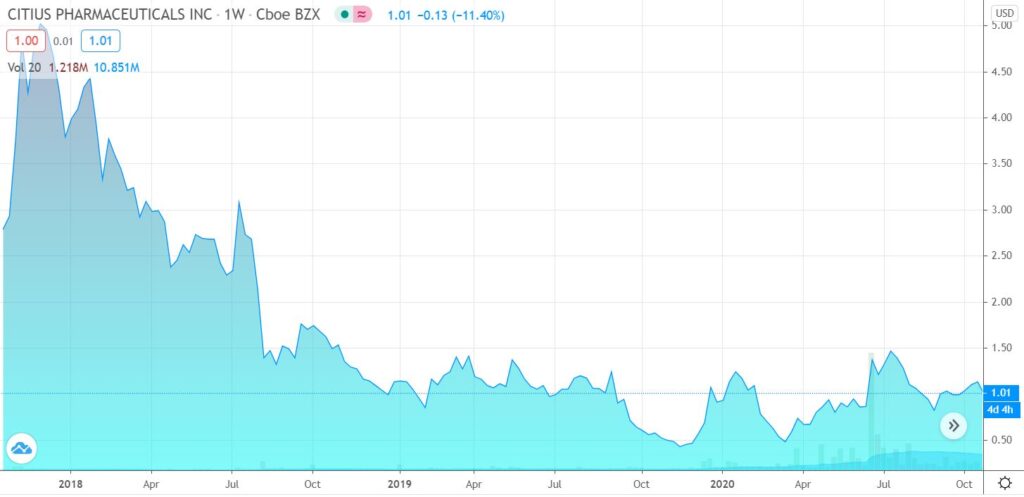
We expect to see significant news in 2021. We recommend reading the most recent shareholder letter (below), but here is our summary re-cap for the attention span disabled!
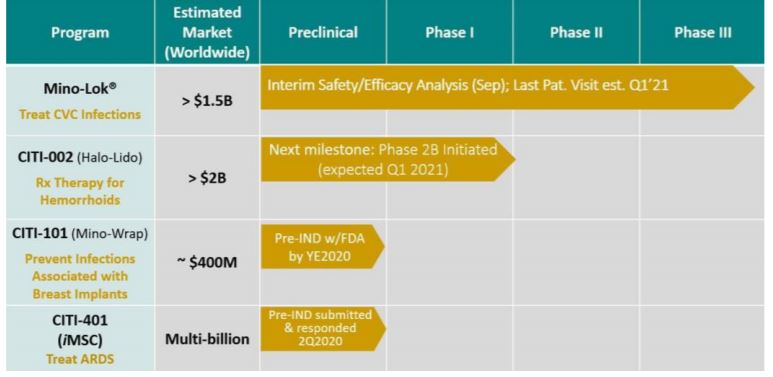
- Mini-Lok. We’re past waiting for results from the Pre-Clinical trial, way past the time-consuming Phase I, Phase II, and three quarters into Phase III – with flying colors and ‘gentleman proceed’ to date.
Note, throughout the Phase III trial, they have had an independent Data Monitoring Committee (DMC) that reviews all the data that came out with patients to date. They can say “beautiful, keep going” or “we “think you should modify the study” or lastly “whoa, stop-halt-no mas. This ain’t working, halt the trial NOW!”
It’s not important for us to try and pretend we have a medical degree and analyze the data. That’s what the DMC is for. Our job is to get excited (and maybe buy more) if they nod their head up and down, meaning yes continue. Or take a bullet or hit the bid, if their head goes side-to-side, meaning no. The most recent ‘continue’ verdict came 9/29/20.
Citius expects the Phase III trial will continue as planned until it is fully enrolled which they expect to be in the first half of 2021. They could have been fully enrolled sooner, but the pandemic has slowed down all clinical trials. One caution though. At the September meeting, they encouraged Citius to determine if the trial will meet the interim superiority threshold at 69 events, and therefore be able to be halted earlier than anticipated. As in ‘before’ the first half of 2021.
So you’ve been warned. Don’t be like some investors who saw Immunomedics double the day they had their trial halted early – waiting for the day.
We could go on about the upcoming milestones for Halo-Lido, Mino-Wrap, and their Novellus/Novacite ARDS for CornaVirus trial milestones, but it’s best if you read it in the shareholder letter below.
Please don’t confuse our lack of reporting on Novellus for a lack of enthusiasm. It has massive potential but we’re laser-focused on Mino-Lok at the moment. Note also Citius is equally enthusiastic, voting with their checkbook to the tune of $5 million in upfront cash, paid last month.
Client, see reports for disclaimer and disclosure details.













