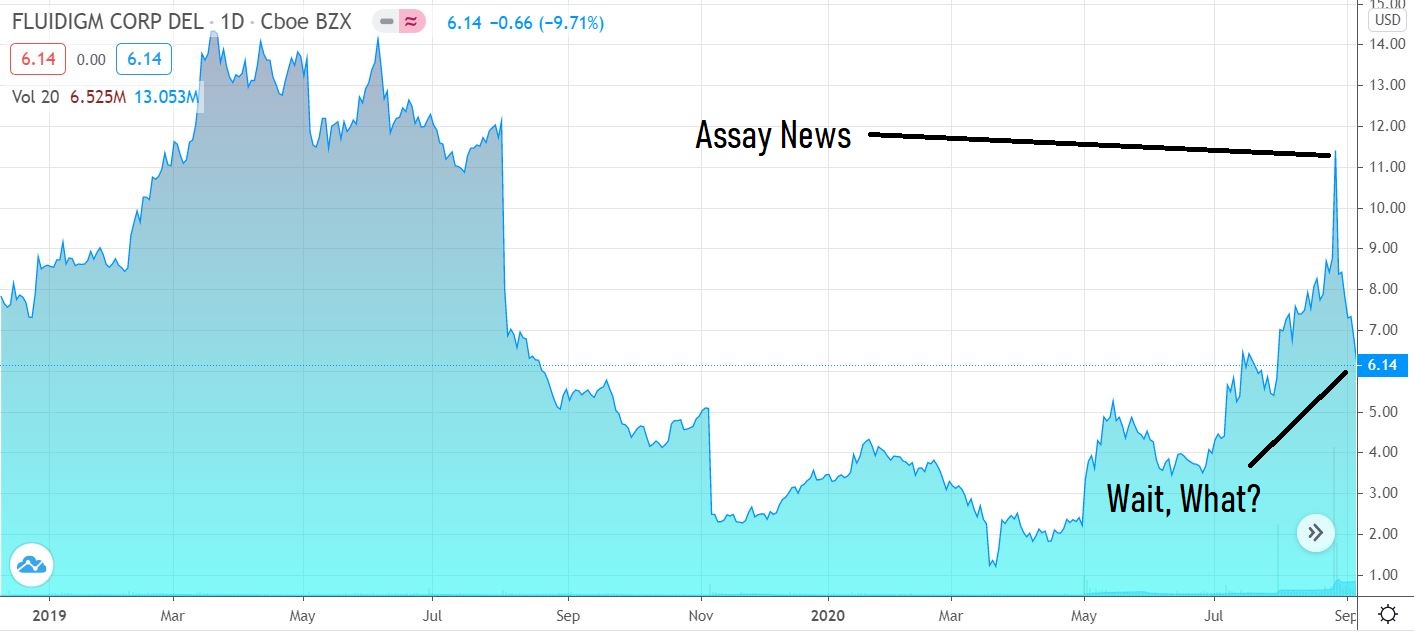
And we have our first ‘bounce’ candidate of the dip..
While Citron Research who’ve we’ve know for like 20 years (they are that good) has a low opinion of Inovio (INO) with a $1.00 price target they seemed wildly bullish on Fluidigm with a $35 target – at least last week they did, on the heels of Corona test kit related news.
THE NEWS:
Fluidigm Granted FDA Emergency Use Authorization for Saliva-Based Assay for COVID-19.
- Easy-to-Administer Saliva Test for COVID-19 Available for Immediate Shipment
- Extraction-Free, Real-Time PCR Workflow with Capacity of up to 6,000 Tests per Day per System
- Demonstrated 100 Percent Agreement with Authorized Nasopharyngeal Assays
SOUTH SAN FRANCISCO, Calif., Aug. 25, 2020 (GLOBE NEWSWIRE) — Fluidigm Corporation (Nasdaq:FLDM), an innovative biotechnology tools provider with a vision to improve life through comprehensive health insight, today announced it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the Advanta™ Dx SARS-CoV-2 RT-PCR Assay, an extraction-free saliva-based test to detect nucleic acid from the SARS‑CoV‑2 virus, designed to be run on the Fluidigm® Biomark™ HD microfluidics platform.
Because it is saliva-based, the Advanta Dx SARS-CoV-2 RT-PCR Assay does not require collection via invasive nasopharyngeal swab. The clinical study associated with the EUA submission demonstrated 100 percent agreement between the saliva results from the Advanta Dx SARS-CoV-2 RT-PCR Assay and the results from paired nasopharyngeal samples tested with authorized assays.
“Accessible and accurate testing programs that include a non-invasive, saliva-based collection option will be essential throughout duration of the COVID-19 pandemic”, said Andrew Lukowiak Ph.D, CEO of San Diego-based Millennium Health, one of several high-complexity labs certified under the Clinical Laboratory Improvement Amendments (CLIA) in the United States that has been evaluating the Advanta Dx SARS-CoV-2 RT-PCR Assay in advance of authorization.
THE STREET:
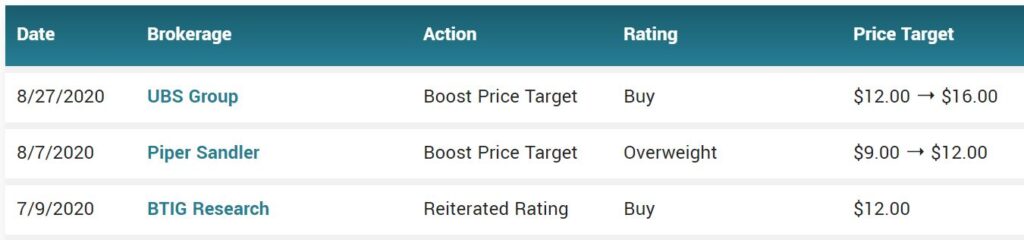
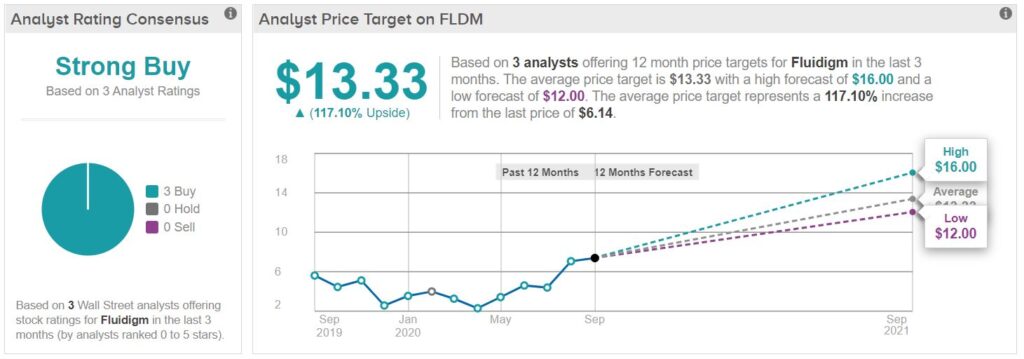
CITRON RESEARCH: