- Mental Health and Wellness Incubator Set to Launch Four Initiatives.
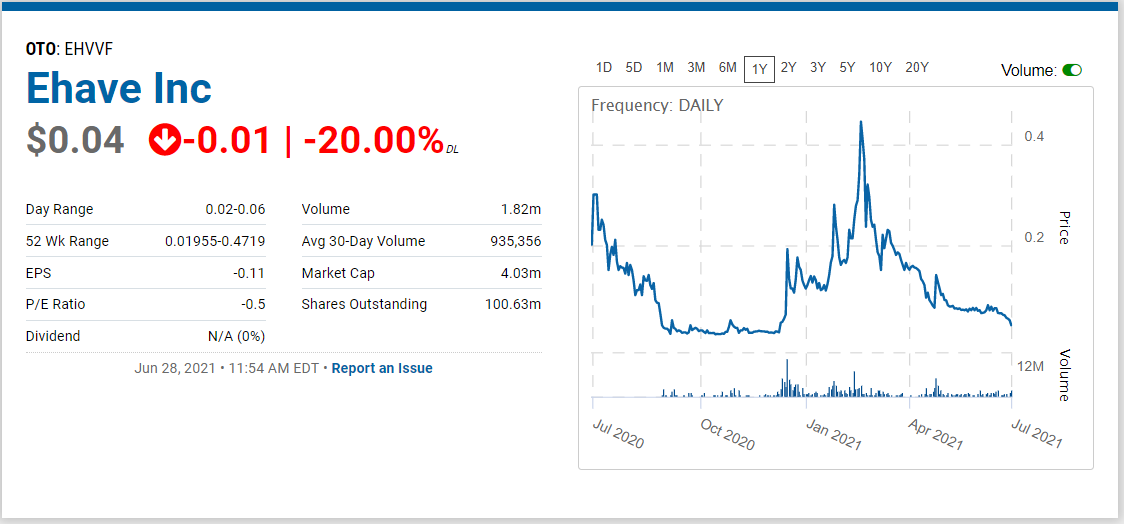
- Trading at Year Low.
- Launching Clinical Trial With Brain Scientific to Monitor Ketamine Efficacy.
- Launching Clinical Trial With Mycotopia Therapies and PsyBioMed.
- Launching KetaDash for At Home Ketamine Treatment.
- Launching Ehave Mobile App for Patient Electronic Health Record.
- Launching Ehave Medical Passport.
LIVE QUOTE
EHAVE INC (EHVVF)

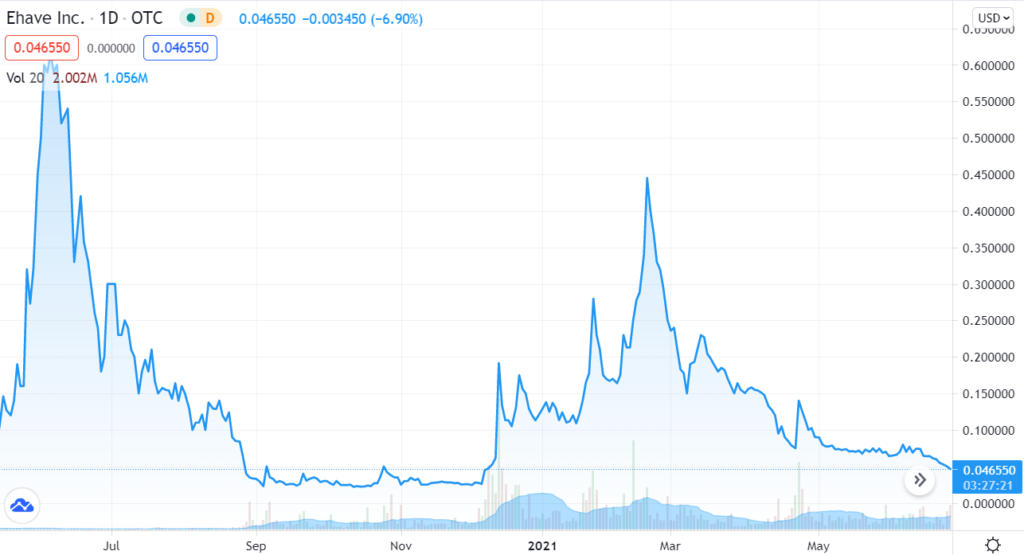
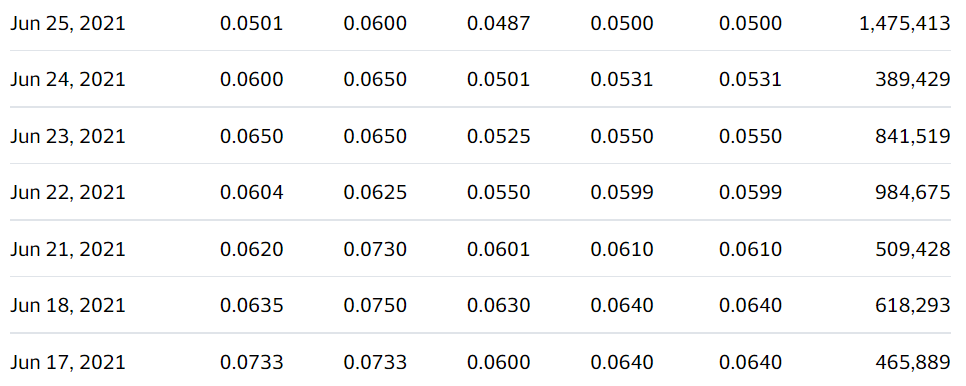
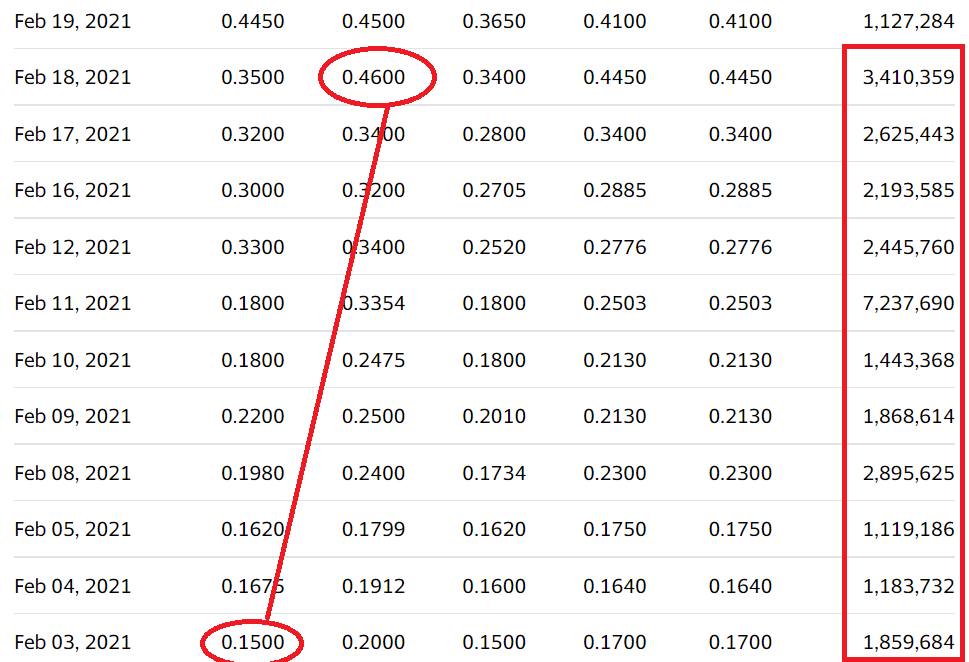
We’re adding Ehave Inc. (EHVFF) to the Watch List to catch a small air-pocket this morning, which may be temporary in our opinion. After a rough quarter with the shares sliding from $0.45 in late February, it traded down a penny to $0.04 this morning.
We are price and time-stamping our interest at $0.04 in what may be the final wash-out day. Don’t tell two friends, until you get in. Trading isn’t active enough to permit acquiring a significant stake (be the bid if you’d like it) – but mum for now!
WHEN SHE RUNS, SHE RUNS!
We’d like to sign them as a client with a long-term outlook for significant gains over the next two to three years. For now, here are some news releases which caught our eye and in the above bullet list.
RELATED: That’s it. $225 Million Raised by Atai (ATAI) Gets us Interested in the Psychedelics Sector.
- Launching Clinical Trial With Brain Scientific to Monitor Ketamine Efficacy.
Announced today plans to launch brain mapping ketamine clinical trials using Brain Scientific technology later this year. The clinical trial will establish the statistical correlation between the ketamine treatment and patient improvement from the disorders. Read More.
- Launching Clinical Trial With Mycotopia Therapies and PsyBioMed.
Announced plans to launch brain mapping ketamine clinical trials using Brain Scientific technology later this year. The clinical trial will establish the statistical correlation between the ketamine treatment and patient improvement from the disorders. The trial, which is designed to enroll 35 patients, is expected to start in the second half of 2021. Ehave is currently completing the necessary preclinical studies necessary to begin enrolling patients in the trial. Read More.
- Launching KetaDash for At Home Ketamine Treatment.
Announced plans to launch open testing of its KetaDASH ketamine IV therapy in the second quarter using advanced vein technology. KetaDASH is a personalized mental care platform designed to instantly connect patients with highly skilled nurses to provide ketamine therapy at home under expert supervision. The KetaDASH platform allows licensed ketamine clinics and patients who have been prescribed ketamine by a physician to administer the treatment at home intravenously. This gives the clinic an opportunity to increase revenues by treating patients who are unable to come to their office. Read More.
- Launching Ehave Mobile App for Patient Electronic Health Record.
Announced the expansion of its Dashboard capabilities to include data management which allow digitally captured information, like electronic health records (EHRs) to be shared. Under the terms of the licensing agreement with Health Wizz, Ehave will offer its dashboard users medical records management. This takes the Ehave dashboard to a new level by providing a HIPAA compliant, decentralized mobile platform that enables people to aggregate, organize and share personal medical health records securely and efficiently on their mobile phone. CEO Benjamin Kaplan said, “We believe people must be in control of their health records. It is their body and their data.” Read More.
- Launching Ehave Medical Passport.
Announced its Ehave medical passport will give small businesses a tool to verify Covid-19 vaccination as most small businesses battle to come to terms on how to implement safety precautions. Ehave’s Medical and Vaccine Passport will allow individuals and small businesses to easily access vaccination verification to ease implementing new safety precautions. As a result of its recent licensing agreement with Health Wizz, Ehave now has the necessary access to provide Medical and Vaccine Passports to those individuals who want to have one. The number of potential participants who can access their health data with the Ehave Mobile App is more than 150 million potential users. Read More.
CURRENT PSYCHEDELIC UNIVERSE RANKED BY MARKET CAP
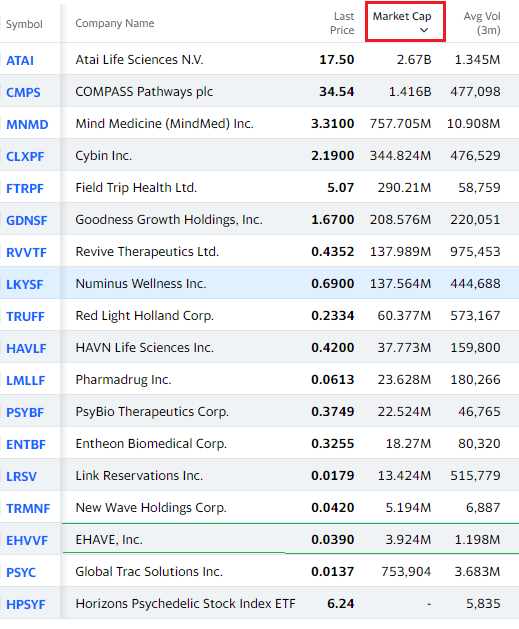
Ehave (EHVVF): “How We Entered the Psychedelic Space.”
SUBSCRIBE for our upcoming Psychedelic Stock Sector Picks.
About Ehave, Inc.
Ehave, Inc. (EHVVF) is a leader of digital therapeutics delivering evidence-based therapeutic interventions to patients. Our primary focus is on improving the standard care in therapeutics to prevent or treat brain disorders or diseases through the use of digital therapeutics, independently or together, with medications, devices, and other therapies to optimize patient care and health outcomes. Our main product is the Ehave Telemetry Portal, which is a mental health informatics platform that allows clinicians to make objective and intelligent decisions through data insights. The Ehave Infinity Portal offers a powerful machine learning and artificial intelligence platform with a growing set of advanced tools and applications developed by Ehave and its leading partners. This empowers patients, healthcare providers, and payers to address a wide range of conditions through high quality, safe, and effective data-driven involvement with intelligent and accessible tools. Additional information on Ehave can be found on the Company’s website at: www.ehave.com .
Forward-Looking Statement Disclaimer
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements: (i) the initiation, timing, progress and results of the Company’s research, manufacturing and other development efforts; (ii) the Company’s ability to advance its products to successfully complete development and commercialization; (iii) the manufacturing, development, commercialization, and market acceptance of the Company’s products; (iv) the lack of sufficient funding to finance the product development and business operations; (v) competitive companies and technologies within the Company’s industry and introduction of competing products; (vi) the Company’s ability to establish and maintain corporate collaborations; (vii) loss of key management personnel; (viii) the scope of protection the Company is able to establish and maintain for intellectual property rights covering its products and its ability to operate its business without infringing the intellectual property rights of others; (ix) potential failure to comply with applicable health information privacy and security laws and other state and federal privacy and security laws; and (x) the difficulty of predicting actions of the USA FDA and its regulations. All forward-looking statements included in this press release are made only as of the date of this press release. The Company assumes no obligation to update any written or oral forward-looking statement unless required by law. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is contained under the heading “Risk Factors” in Ehave, Inc.’s Registration Statement on Form F-1 filed with the Securities and Exchange Commission (SEC) on September 24, 2015, as amended, which is available on the SEC’s website, http://www.sec.gov. Not a client, though negotiating a relationship.