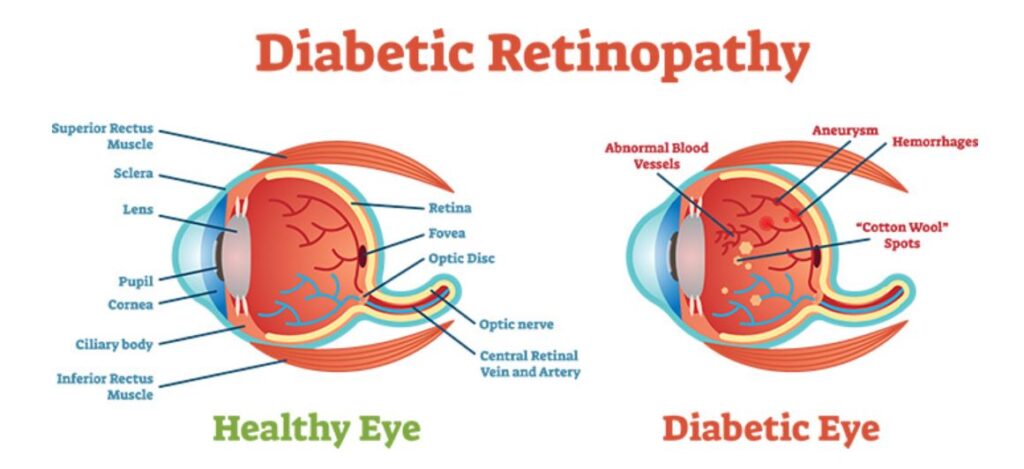
New client and an incubator, studying now. Thinly, thinly traded. We like what we’ve read so far about their vitreous substitute (eyeball fluid) called Vitargus®, which is used as a biodegradable vitreous substitute in patients under vitrectomy surgery for retinal detachment or vitreous hemorrhage. Big market for the treatment of post-surgical diabetic retinopathy (DR). Worldwide, one-third of the estimated 285 million people with diabetes show signs of DR. Hmm.
“One of the earliest milestones expected to be achieved is the licensing of ABVC’s innovative vitreous substitute, Vitargus, to one or more marketing partners. Since it has demonstrated in clinical trials that, compared with other currently used methods, patients undergoing surgery to re-attach the retina through Vitargus are able to recover more quickly and experience little side effects to retinal tissue and vision. Also, because Vitargus is bio-degradable, there is no need for a second surgery.” Dr. Howard Doong, the CEO of American BriVision.
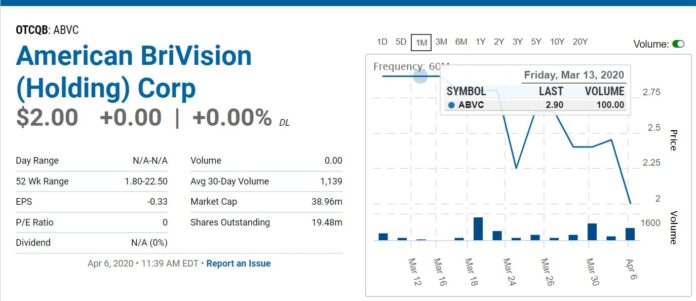
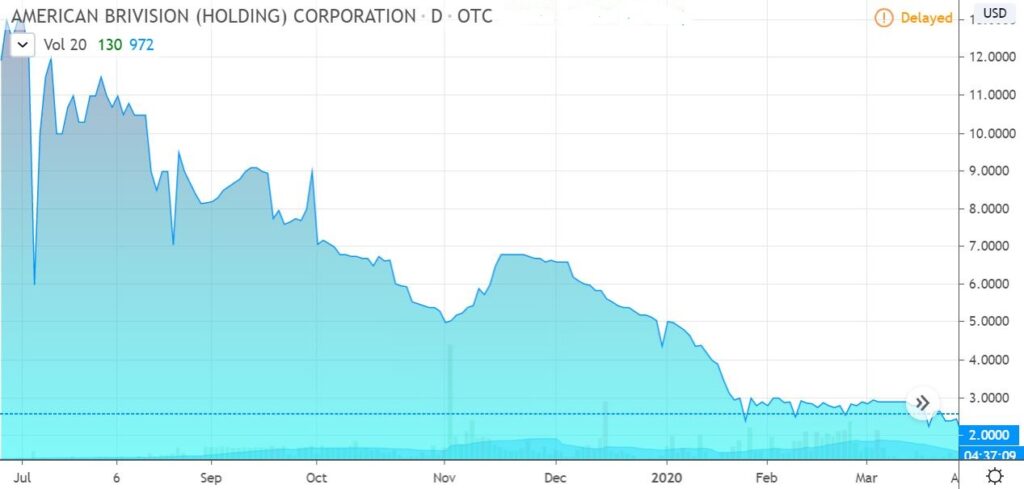
Chart looks nice for bargain hunters!
Sign Up For American BriVision News Here
American BriVision Provides Corporate Update
FREMONT, CA, Feb. 28, 2020 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — American BriVision (Holding) Corporation (OTCQB: ABVC), an emerging clinical stage biopharmaceutical company focused on commercializing promising new drugs developed by world-class research institutions such as Sloan Kettering and Stanford University, today provided a corporate update of recent medical accomplishments.
The Company’s highlights include:
- Encouraging results announced from First-in-Human (Feasibility) Clinical Trial for Vitargus®
- Completion of a Phase II clinical study for Major Depressive Disorder with ABV-1504 and the release of the full Clinical Study
- Initiating a Phase II Part I clinical trial for Adult Attention-Deficit Hyperactivity Disorder with ABV-1505
ABVC Medical Device Development in 2019
ABV-1701/Vitargus® is a Vitreous Substitute for Vitrectomy Surgery
A First-in-Human (Feasibility) clinical study of ABV-1701, or Vitargus®, used as a biodegradable vitreous substitute in patients under vitrectomy surgery for retinal detachment or vitreous hemorrhage, was successfully completed at the Sydney Eye Hospital in Australia. The feasibility clinical study results were reported by Dr. Andrew Chang, the principal investigator, at the Retina Subspecialty Day program of the American Academy of Ophthalmology (AAO) 2019 Annual Meeting in San Francisco on October 11th.
The study showed Vitargus® was generally well-tolerated and effective as a vitreous substitute, and no apparent toxicity or serious adverse events (SAEs) directly caused by Vitargus® were observed. Moreover, an exploratory analysis showed a statistically significant improvement in best corrected visual acuity (BCVA) from the baseline. Given the encouraging study results, a multi-national, multi-site pivotal study for Vitargus® is planned in 2020.
ABVC Drug Development Achievements in 2019
ABV-1504 in Major Depressive Disorder (“MDD”)
A Phase II clinical study – pursuant to U.S. Food and Drug Administration (FDA) and Taiwan FDA (TFDA) clinical protocol code BLI-1005-002 was successfully completed by Stanford University and five major medical centers in Taiwan. A full clinical study report (CSR) was submitted to the FDA and TFDA on the FDA (on 2019/12/4) and TFDA (on 2019/12/5).
Capsules (2 x 380 mg) of PDC-1421, the active pharmaceutical ingredient in ABV-1504, met the prespecified primary objective by demonstrating a highly significant 13.2-point reduction in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score in Intention-To-Treat (ITT) population, averaged over the 6-week treatment period from the baseline. PDC-1421 regimens were safe and well tolerated with no serious adverse events. The positive Phase II results of lead drug candidate PDC-1421 for MDD will enable ABVC to initiate End-of-Phase II meetings (EOP2) with the FDA and TFDA regulatory authorities to discuss Phase III trial design for ABV-1504, NDA-related plans, and an eventual road map for market launch.
ABV-1505 in Adult Attention-Deficit Hyperactivity Disorder (“ADHD”)
A Phase II Part I clinical trial, under FDA clinical protocol code BLI-1008-001 for adult attention-deficit hyperactivity disorder (ADHD), was initiated at the University of California San Francisco (UCSF) Medical Center in the fall of 2019.
The primary objective of this study is to determine the effective doses and treatment period of PDC-1421, the active pharmaceutical ingredient in ABV-1505, in adult patients with ADHD. The secondary objective is to evaluate the safety of PDC-1421 in patients receiving the drug at various dose levels. A Phase II Part II study is expected to follow at the UCSF Medical Center, along with major medical centers in Taiwan, after and subject to successful completion of this Phase II Part I study. The Phase II clinical study with ABV-1505 for the treatment of adult ADHD patients is aiming to further expand PDC-1421’s therapeutic indication.
ABVC Products Address Several Significant Billion Dollar Markets
Vitargus®, ABVC’s ophthalmological medical device now in development, is targeted at the $1.8 billion (in 2019) retinal vitrectomy market. ABV-1504, a drug now under development would be used in the major depressive disorder drug market, which was a $6.5B worldwide market in 2017. Additionally, ABV-1505, a drug now being evaluated in the ADHD drug market, had a $17.2B worldwide market in 2018.
Dr. Howard Doong, CEO of American BriVision, commented, “We are excited to have clinical results from the proof-of-concept studies of our drug products and medical device, and we believe the results of these studies will advance our product pipeline in 2020.”
About American BriVision
American BriVision is a clinical stage biopharmaceutical company focused on utilizing its licensed technology to conduct proof-of-concept trials through Phase II of the clinical development process at world-famous research institutions (such as Stanford University, University of California at San Francisco, and Cedars-Sinai Medical Center). The company has an active pipeline of six drugs and one medical device (ABV-1701/Vitargus®) under development.
CAUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMENTS
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the possibility of unfavorable terms for planned financing and the possibility that we may be unable to complete the up-listing in the currently anticipated timelines or at all, that the possibility of unfavorable results from ongoing and additional clinical trials involving Vitargus and the possibility that we may be unable to complete one or more of such trials in the currently anticipated timelines or at all. Further, it is possible that the parties may make a strategic decision to discontinue development of Vitargus, and as a result, Vitargus may never be successfully commercialized. We base these forward-looking statements on our expectations and projections about future events, which we derive from the information currently available to us. Such forward-looking statements relate to future events or our future performance, including: our financial performance and projections; our growth in revenue and earnings; and our business prospects and opportunities. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including: our ability to change the direction of the Company; our ability to keep pace with new technology and changing market needs; our having adequate funding to conduct our clinical trials; and the competitive environment of our business. These and other factors may cause our actual results to differ materially from any forward-looking statement. Forward-looking statements are only predictions. The forward-looking events discussed in this document and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions about us. We are not obligated to publicly update or revise any forward-looking statement, whether as a result of uncertainties and assumptions, the forward-looking events discussed in this document, and other statements made from time to time by us or our representatives that might occur.
Contact:
Andy An – Chief Financial Officer
765-610-8826
https://www.ldmicro.com/profile/abvc/news/7583035860700056
Client, see upcoming report for disclosure and disclaimer details.